Endogenous hepcidin and its agonist mediate resistance to selected infections by clearing non-transferrin-bound iron
- PMID: 28465342
- PMCID: PMC5520472
- DOI: 10.1182/blood-2017-03-772715
Endogenous hepcidin and its agonist mediate resistance to selected infections by clearing non-transferrin-bound iron
Abstract
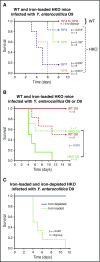
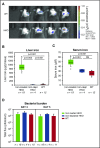
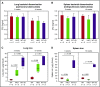
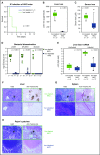
The iron-regulatory hormone hepcidin is induced early in infection, causing iron sequestration in macrophages and decreased plasma iron; this is proposed to limit the replication of extracellular microbes, but could also promote infection with macrophage-tropic pathogens. The mechanisms by which hepcidin and hypoferremia modulate host defense, and the spectrum of microbes affected, are poorly understood. Using mouse models, we show that hepcidin was selectively protective against siderophilic extracellular pathogens (Yersinia enterocolitica O9) by controlling non-transferrin-bound iron (NTBI) rather than iron-transferrin concentration. NTBI promoted the rapid growth of siderophilic but not nonsiderophilic bacteria in mice with either genetic or iatrogenic iron overload and in human plasma. Hepcidin or iron loading did not affect other key components of innate immunity, did not indiscriminately promote intracellular infections (Mycobacterium tuberculosis), and had no effect on extracellular nonsiderophilic Y enterocolitica O8 or Staphylococcus aureus Hepcidin analogs may be useful for treatment of siderophilic infections.
© 2017 by The American Society of Hematology.
Figures
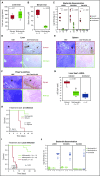

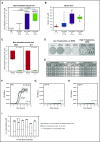
Comment in
-
Ironing out the role of hepcidin in infection.Blood. 2017 Jul 20;130(3):233-234. doi: 10.1182/blood-2017-05-783688. Blood. 2017. PMID: 28729331 No abstract available.
References
-
- Nemeth E, Ganz T. Regulation of iron metabolism by hepcidin. Annu Rev Nutr. 2006;26:323-342. - PubMed
-
- Christopher GW. Escherichia coli bacteremia, meningitis, and hemochromatosis. Arch Intern Med. 1985;145(10):1908. - PubMed
-
- Gerhard GS, Levin KA, Price Goldstein J, Wojnar MM, Chorney MJ, Belchis DA. Vibrio vulnificus septicemia in a patient with the hemochromatosis HFE C282Y mutation. Arch Pathol Lab Med. 2001;125(8):1107-1109. - PubMed
-
- Manso C, Rivas I, Peraire J, Vidal F, Richart C. Fatal Listeria meningitis, endocarditis and pericarditis in a patient with haemochromatosis. Scand J Infect Dis. 1997;29(3):308-309. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

