Myostatin inhibits osteoblastic differentiation by suppressing osteocyte-derived exosomal microRNA-218: A novel mechanism in muscle-bone communication
- PMID: 28465350
- PMCID: PMC5491785
- DOI: 10.1074/jbc.M116.770941
Myostatin inhibits osteoblastic differentiation by suppressing osteocyte-derived exosomal microRNA-218: A novel mechanism in muscle-bone communication
Abstract
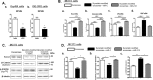
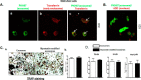
Muscle and bone are closely associated in both anatomy and function, but the mechanisms that coordinate their synergistic action remain poorly defined. Myostatin, a myokine secreted by muscles, has been shown to inhibit muscle growth, and the disruption of the myostatin gene has been reported to cause muscle hypertrophy and increase bone mass. Extracellular vesicle-exosomes that carry microRNA (miRNA), mRNA, and proteins are known to perform an important role in cell-cell communication. We hypothesized that myostatin may play a crucial role in muscle-bone interactions and may promote direct effects on osteocytes and on osteocyte-derived exosomal miRNAs, thereby indirectly influencing the function of other bone cells. We report herein that myostatin promotes expression of several bone regulators such as sclerostin (SOST), DKK1, and RANKL in cultured osteocytic (Ocy454) cells, concomitant with the suppression of miR-218 in both parent Ocy454 cells and derived exosomes. Exosomes produced by Ocy454 cells that had been pretreated with myostatin could be taken up by osteoblastic MC3T3 cells, resulting in a marked reduction of Runx2, a key regulator of osteoblastic differentiation, and in decreased osteoblastic differentiation via the down-regulation of the Wnt signaling pathway. Importantly, the inhibitory effect of myostatin-modified osteocytic exosomes on osteoblast differentiation is completely reversed by expression of exogenous miR-218, through a mechanism involving miR-218-mediated inhibition of SOST. Together, our findings indicate that myostatin directly influences osteocyte function and thereby inhibits osteoblastic differentiation, at least in part, through the suppression of osteocyte-derived exosomal miR-218, suggesting a novel mechanism in muscle-bone communication.
Keywords: Wnt signaling; bone; exosome (vesicle); microRNA (miRNA); myostatin; osteoblast; osteocyte; skeletal muscle metabolism.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures





References
-
- Schoenau E. (2005) From mechanostat theory to development of the “Functional Muscle-Bone-Unit.” J. Musculoskelet. Neuronal Interact. 5, 232–238 - PubMed
-
- Bloomfield S. A. (2010) Disuse osteopenia. Curr. Osteoporos. Rep. 8, 91–97 - PubMed
-
- Karinkanta S., Piirtola M., Sievänen H., Uusi-Rasi K., and Kannus P. (2010) Physical therapy approaches to reduce fall and fracture risk among older adults. Nat. Rev. Endocrinol. 6, 396–407 - PubMed
-
- Rittweger J., Beller G., Ehrig J., Jung C., Koch U., Ramolla J., Schmidt F., Newitt D., Majumdar S., Schiessl H., and Felsenberg D. (2000) Bone-muscle strength indices for the human lower leg. Bone 27, 319–326 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

