Tetramethylpyrazine blocks TFAM degradation and up-regulates mitochondrial DNA copy number by interacting with TFAM
- PMID: 28465355
- PMCID: PMC5434891
- DOI: 10.1042/BSR20170319
Tetramethylpyrazine blocks TFAM degradation and up-regulates mitochondrial DNA copy number by interacting with TFAM
Abstract
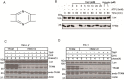
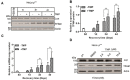
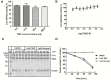
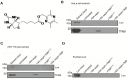
The natural small molecule compound: 2,3,5,6-tetramethylpyrazine (TMP), is a major component of the Chinese medicine Chuanxiong, which has wide clinical applications in dilating blood vessels, inhibiting platelet aggregation and treating thrombosis. Recent work suggests that TMP is also an antitumour agent. Despite its chemotherapeutic potential, the mechanism(s) underlying TMP action are unknown. Herein, we demonstrate that TMP binds to mitochondrial transcription factor A (TFAM) and blocks its degradation by the mitochondrial Lon protease. TFAM is a key regulator of mtDNA replication, transcription and transmission. Our previous work showed that when TFAM is not bound to DNA, it is rapidly degraded by the ATP-dependent Lon protease, which is essential for mitochondrial proteostasis. In cultured cells, TMP specifically blocks Lon-mediated degradation of TFAM, leading to TFAM accumulation and subsequent up-regulation of mtDNA content in cells with substantially low levels of mtDNA. In vitro protease assays show that TMP does not directly inhibit mitochondrial Lon, rather interacts with TFAM and blocks degradation. Pull-down assays show that biotinylated TMP interacts with TFAM. These findings suggest a novel mechanism whereby TMP stabilizes TFAM and confers resistance to Lon-mediated degradation, thereby promoting mtDNA up-regulation in cells with low mtDNA content.
Keywords: Lon protease; Tetramethylpyrazine; mitochondria; mitochondrial DNA; mitochondrial transcription factor A.
© 2017 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures
Similar articles
-
Phosphorylation of human TFAM in mitochondria impairs DNA binding and promotes degradation by the AAA+ Lon protease.Mol Cell. 2013 Jan 10;49(1):121-32. doi: 10.1016/j.molcel.2012.10.023. Epub 2012 Nov 29. Mol Cell. 2013. PMID: 23201127 Free PMC article.
-
Mitochondrial transcription factor A regulates mitochondrial transcription initiation, DNA packaging, and genome copy number.Biochim Biophys Acta. 2012 Sep-Oct;1819(9-10):921-9. doi: 10.1016/j.bbagrm.2012.03.002. Epub 2012 Mar 21. Biochim Biophys Acta. 2012. PMID: 22465614 Review.
-
Transient overexpression of mitochondrial transcription factor A (TFAM) is sufficient to stimulate mitochondrial DNA transcription, but not sufficient to increase mtDNA copy number in cultured cells.Nucleic Acids Res. 2004 Nov 16;32(20):6015-27. doi: 10.1093/nar/gkh921. Print 2004. Nucleic Acids Res. 2004. PMID: 15547250 Free PMC article.
-
Mitochondrial Lon protease regulates mitochondrial DNA copy number and transcription by selective degradation of mitochondrial transcription factor A (TFAM).Proc Natl Acad Sci U S A. 2010 Oct 26;107(43):18410-5. doi: 10.1073/pnas.1008924107. Epub 2010 Oct 7. Proc Natl Acad Sci U S A. 2010. PMID: 20930118 Free PMC article.
-
Mitochondrial pathways to cardiac recovery: TFAM.Heart Fail Rev. 2016 Sep;21(5):499-517. doi: 10.1007/s10741-016-9561-8. Heart Fail Rev. 2016. PMID: 27166683 Free PMC article. Review.
Cited by
-
Higher proteotoxic stress rather than mitochondrial damage is involved in higher neurotoxicity of bortezomib compared to carfilzomib.Redox Biol. 2020 May;32:101502. doi: 10.1016/j.redox.2020.101502. Epub 2020 Mar 21. Redox Biol. 2020. PMID: 32244176 Free PMC article.
-
Harnessing mitochondrial biogenesis to combat acute kidney injury: Current insights and futuredirections.Genes Dis. 2025 Apr 15;12(6):101645. doi: 10.1016/j.gendis.2025.101645. eCollection 2025 Nov. Genes Dis. 2025. PMID: 40821125 Free PMC article. Review.
-
CKIP-1 silencing suppresses OSCC via mitochondrial homeostasis-associated TFAM/cGAS-STING signalling axis.J Cell Mol Med. 2024 Aug;28(16):e70006. doi: 10.1111/jcmm.70006. J Cell Mol Med. 2024. PMID: 39169452 Free PMC article.
-
Miriplatin-loaded liposome, as a novel mitophagy inducer, suppresses pancreatic cancer proliferation through blocking POLG and TFAM-mediated mtDNA replication.Acta Pharm Sin B. 2023 Nov;13(11):4477-4501. doi: 10.1016/j.apsb.2023.07.009. Epub 2023 Jul 16. Acta Pharm Sin B. 2023. PMID: 37969736 Free PMC article.
-
Mitochondrial DNA stress triggers autophagy-dependent ferroptotic death.Autophagy. 2021 Apr;17(4):948-960. doi: 10.1080/15548627.2020.1739447. Epub 2020 Mar 18. Autophagy. 2021. PMID: 32186434 Free PMC article.
References
-
- Goard C.A. and Schimmer A.D. (2014) Mitochondrial matrix proteases as novel therapeutic targets in malignancy. Oncogene 33, 2690–2699 - PubMed
-
- Granot Z., Kobiler O., Melamed-Book N., Eimerl S., Bahat A., Lu B. et al. (2007) Turnover of mitochondrial steroidogenic acute regulatory (StAR) protein by Lon protease: the unexpected effect of proteasome inhibitors. Mol. Endocrinol. 21, 2164–2177 - PubMed
-
- Bota D.A. and Davies K.J. (2002) Lon protease preferentially degrades oxidized mitochondrial aconitase by an ATP-stimulated mechanism. Nat. Cell Biol. 4, 674–680 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

