A Novel Agonist of the TRIF Pathway Induces a Cellular State Refractory to Replication of Zika, Chikungunya, and Dengue Viruses
- PMID: 28465426
- PMCID: PMC5414005
- DOI: 10.1128/mBio.00452-17
A Novel Agonist of the TRIF Pathway Induces a Cellular State Refractory to Replication of Zika, Chikungunya, and Dengue Viruses
Abstract
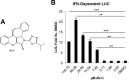
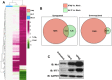
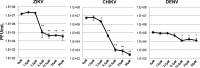
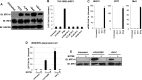
The ongoing concurrent outbreaks of Zika, Chikungunya, and dengue viruses in Latin America and the Caribbean highlight the need for development of broad-spectrum antiviral treatments. The type I interferon (IFN) system has evolved in vertebrates to generate tissue responses that actively block replication of multiple known and potentially zoonotic viruses. As such, its control and activation through pharmacological agents may represent a novel therapeutic strategy for simultaneously impairing growth of multiple virus types and rendering host populations resistant to virus spread. In light of this strategy's potential, we undertook a screen to identify novel interferon-activating small molecules. Here, we describe 1-(2-fluorophenyl)-2-(5-isopropyl-1,3,4-thiadiazol-2-yl)-1,2-dihydrochromeno[2,3-c]pyrrole-3,9-dione, which we termed AV-C. Treatment of human cells with AV-C activates innate and interferon-associated responses that strongly inhibit replication of Zika, Chikungunya, and dengue viruses. By utilizing genome editing, we investigated the host proteins essential to AV-C-induced cellular states. This showed that the compound requires a TRIF-dependent signaling cascade that culminates in IFN regulatory factor 3 (IRF3)-dependent expression and secretion of type I interferon to elicit antiviral responses. The other canonical IRF3-terminal adaptor proteins STING and IPS-1/MAVS were dispensable for AV-C-induced phenotypes. However, our work revealed an important inhibitory role for IPS-1/MAVS, but not TRIF, in flavivirus replication, implying that TRIF-directed viral evasion may not occur. Additionally, we show that in response to AV-C, primary human peripheral blood mononuclear cells secrete proinflammatory cytokines that are linked with establishment of adaptive immunity to viral pathogens. Ultimately, synthetic innate immune activators such as AV-C may serve multiple therapeutic purposes, including direct antimicrobial responses and facilitation of pathogen-directed adaptive immunity.IMPORTANCE The type I interferon system is part of the innate immune response that has evolved in vertebrates as a first line of broad-spectrum immunological defense against an unknowable diversity of microbial, especially viral, pathogens. Here, we characterize a novel small molecule that artificially activates this response and in so doing generates a cellular state antagonistic to growth of currently emerging viruses: Zika virus, Chikungunya virus, and dengue virus. We also show that this molecule is capable of eliciting cellular responses that are predictive of establishment of adaptive immunity. As such, this agent may represent a powerful and multipronged therapeutic tool to combat emerging and other viral diseases.
Keywords: antiviral agents; emerging virus; innate immunity; interferons.
Copyright © 2017 Pryke et al.
Figures
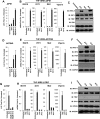
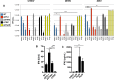

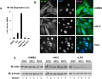
References
-
- Roth A, Mercier A, Lepers C, Hoy D, Duituturaga S, Benyon E, Guillaumot L, Souares Y. 2014. Concurrent outbreaks of dengue, Chikungunya and Zika virus infections—an unprecedented epidemic wave of mosquito-borne viruses in the Pacific 2012–2014. Euro Surveill 19:pii=20929. doi: 10.2807/1560-7917.ES2014.19.41.20929. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
