Molecular codes for cell type specification in Brn3 retinal ganglion cells
- PMID: 28465430
- PMCID: PMC5441800
- DOI: 10.1073/pnas.1618551114
Molecular codes for cell type specification in Brn3 retinal ganglion cells
Abstract
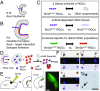
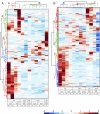
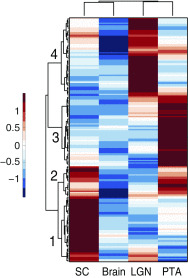
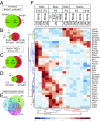
Visual information is conveyed from the eye to the brain by distinct types of retinal ganglion cells (RGCs). It is largely unknown how RGCs acquire their defining morphological and physiological features and connect to upstream and downstream synaptic partners. The three Brn3/Pou4f transcription factors (TFs) participate in a combinatorial code for RGC type specification, but their exact molecular roles are still unclear. We use deep sequencing to define (i) transcriptomes of Brn3a- and/or Brn3b-positive RGCs, (ii) Brn3a- and/or Brn3b-dependent RGC transcripts, and (iii) transcriptomes of retinorecipient areas of the brain at developmental stages relevant for axon guidance, dendrite formation, and synaptogenesis. We reveal a combinatorial code of TFs, cell surface molecules, and determinants of neuronal morphology that is differentially expressed in specific RGC populations and selectively regulated by Brn3a and/or Brn3b. This comprehensive molecular code provides a basis for understanding neuronal cell type specification in RGCs.
Keywords: Pou4f1; Pou4f2; neuronal cell types; retinal ganglion cells; transcription factors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








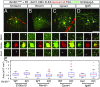

Similar articles
-
Genetic interactions between Brn3 transcription factors in retinal ganglion cell type specification.PLoS One. 2013 Oct 8;8(10):e76347. doi: 10.1371/journal.pone.0076347. eCollection 2013. PLoS One. 2013. PMID: 24116103 Free PMC article.
-
Postnatal developmental dynamics of cell type specification genes in Brn3a/Pou4f1 Retinal Ganglion Cells.Neural Dev. 2018 Jun 29;13(1):15. doi: 10.1186/s13064-018-0110-0. Neural Dev. 2018. PMID: 29958540 Free PMC article.
-
Whole number, distribution and co-expression of brn3 transcription factors in retinal ganglion cells of adult albino and pigmented rats.PLoS One. 2012;7(11):e49830. doi: 10.1371/journal.pone.0049830. Epub 2012 Nov 16. PLoS One. 2012. PMID: 23166779 Free PMC article.
-
A gene regulatory hierarchy for retinal ganglion cell specification and differentiation.Semin Cell Dev Biol. 2004 Feb;15(1):115-23. doi: 10.1016/j.semcdb.2003.09.009. Semin Cell Dev Biol. 2004. PMID: 15036214 Review.
-
What is the nature of the RGC-5 cell line?Adv Exp Med Biol. 2014;801:145-54. doi: 10.1007/978-1-4614-3209-8_19. Adv Exp Med Biol. 2014. PMID: 24664692 Review.
Cited by
-
A non-transcriptional function of Yap regulates the DNA replication program in Xenopus laevis.Elife. 2022 Jul 15;11:e75741. doi: 10.7554/eLife.75741. Elife. 2022. PMID: 35838349 Free PMC article.
-
Essential Roles of Tbr1 in the Formation and Maintenance of the Orientation-Selective J-RGCs and a Group of OFF-Sustained RGCs in Mouse.Cell Rep. 2019 Apr 16;27(3):900-915.e5. doi: 10.1016/j.celrep.2019.03.077. Cell Rep. 2019. PMID: 30995485 Free PMC article.
-
In vivo and in vitro knockout system labelled using fluorescent protein via microhomology-mediated end joining.Life Sci Alliance. 2019 Dec 24;3(1):e201900528. doi: 10.26508/lsa.201900528. Print 2020 Jan. Life Sci Alliance. 2019. PMID: 31874862 Free PMC article.
-
Molecular mechanisms regulating synaptic specificity and retinal circuit formation.Wiley Interdiscip Rev Dev Biol. 2021 Jan;10(1):e379. doi: 10.1002/wdev.379. Epub 2020 Apr 8. Wiley Interdiscip Rev Dev Biol. 2021. PMID: 32267095 Free PMC article. Review.
-
Genetic control of retinal ganglion cell genesis.Cell Mol Life Sci. 2021 May;78(9):4417-4433. doi: 10.1007/s00018-021-03814-w. Epub 2021 Mar 29. Cell Mol Life Sci. 2021. PMID: 33782712 Free PMC article. Review.
References
-
- Hobert O. Regulation of terminal differentiation programs in the nervous system. Annu Rev Cell Dev Biol. 2011;27:681–696. - PubMed
-
- Jessell TM. Neuronal specification in the spinal cord: Inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–29. - PubMed
-
- Komiyama T, Luo L. Development of wiring specificity in the olfactory system. Curr Opin Neurobiol. 2006;16:67–73. - PubMed
-
- Bassett EA, Wallace VA. Cell fate determination in the vertebrate retina. Trends Neurosci. 2012;35:565–573. - PubMed
-
- Badea TC, Nathans J. Quantitative analysis of neuronal morphologies in the mouse retina visualized by using a genetically directed reporter. J Comp Neurol. 2004;480:331–351. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

