Phase I and Preliminary Phase II Study of TRC105 in Combination with Sorafenib in Hepatocellular Carcinoma
- PMID: 28465443
- PMCID: PMC6640149
- DOI: 10.1158/1078-0432.CCR-16-3171
Phase I and Preliminary Phase II Study of TRC105 in Combination with Sorafenib in Hepatocellular Carcinoma
Abstract
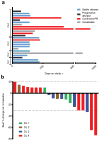
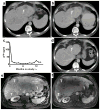
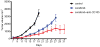
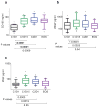
Purpose: Endoglin (CD105) is an endothelial cell membrane receptor highly expressed on proliferating tumor vasculature, including that of hepatocellular carcinoma (HCC), and is associated with poor prognosis. Endoglin is essential for angiogenesis, and its expression is induced by hypoxia and VEGF pathway inhibition. TRC105 is a chimeric IgG1 CD105 mAb that inhibits angiogenesis and causes antibody-dependent cellular cytotoxicity and apoptosis of proliferating endothelium.Experimental Design: Patients with HCC (Child-Pugh A/B7), ECOG 0/1, were enrolled in a phase I study of TRC105 at 3, 6, 10, and 15 mg/kg every 2 weeks given with sorafenib 400 mg twice daily. Correlative biomarkers included DCE-MRI and plasma levels of angiogenic factors, including soluble endoglin. Pharmacokinetics were assessed in serum.Results: Twenty-six patients were enrolled, of whom 25 received treatment, 15 with cirrhosis. Hep B/C: 3/15; M:F 19:6; mean age of 60 (range, 18-76); 1 DLT (grade 3 AST) occurred at 10 mg/kg. The most frequent toxicity was low-grade epistaxis, a known toxicity of TRC105. One patient experienced an infusion reaction and was replaced. One patient with coronary stenosis developed a fatal myocardial infarction, and one patient developed G3 cerebral tumor hemorrhage. MTD was not established and DL4 (15 mg/kg) was expanded. The overall response rate in 24 evaluable patients at all 4 dose levels was 21% [95% confidence interval (CI), 7.1-42.2], and 25% (95% CI, 8.7-49.1) in patients with measureable disease. Four patients had confirmed stable disease, one of whom was treated for 22 months. Median progression-free survival (PFS) for 24 patients evaluable for PFS was 3.8 months (95% CI, 3.2-5.6 months); median overall survival was 15.5 months (95% CI, 8.5-26.3 months).Conclusions: TRC105 combined with sorafenib was well tolerated at the recommended single agent doses of both drugs. Encouraging evidence of activity to date (PR rate 25%) was observed, and the study is now continuing to recruit in the phase II stage as a multicenter study to confirm activity of the combination. Clin Cancer Res; 23(16); 4633-41. ©2017 AACR.
©2017 American Association for Cancer Research.
Conflict of interest statement
Conflict of interest: The authors declare no conflict of interest.
Figures
Similar articles
-
Phase I study investigating everolimus combined with sorafenib in patients with advanced hepatocellular carcinoma.J Hepatol. 2013 Dec;59(6):1271-7. doi: 10.1016/j.jhep.2013.07.029. Epub 2013 Aug 6. J Hepatol. 2013. PMID: 23928403 Clinical Trial.
-
A phase Ib study of selumetinib (AZD6244, ARRY-142886) in combination with sorafenib in advanced hepatocellular carcinoma (HCC).Ann Oncol. 2016 Dec;27(12):2210-2215. doi: 10.1093/annonc/mdw415. Epub 2016 Sep 28. Ann Oncol. 2016. PMID: 27681866 Clinical Trial.
-
Phase I Study of Lenalidomide and Sorafenib in Patients With Advanced Hepatocellular Carcinoma.Oncologist. 2016 Jun;21(6):664-5. doi: 10.1634/theoncologist.2016-0071. Epub 2016 Jun 2. Oncologist. 2016. PMID: 27256874 Free PMC article. Clinical Trial.
-
Temsirolimus combined with sorafenib in hepatocellular carcinoma: a phase I dose-finding trial with pharmacokinetic and biomarker correlates.Ann Oncol. 2013 Jul;24(7):1900-1907. doi: 10.1093/annonc/mdt109. Epub 2013 Mar 21. Ann Oncol. 2013. PMID: 23519998 Free PMC article. Clinical Trial.
-
Adverse events affect sorafenib efficacy in patients with recurrent hepatocellular carcinoma after liver transplantation: experience at a single center and review of the literature.Eur J Gastroenterol Hepatol. 2013 Feb;25(2):180-6. doi: 10.1097/MEG.0b013e328359e550. Eur J Gastroenterol Hepatol. 2013. PMID: 23044808 Review.
Cited by
-
Therapeutic Targets for Bone and Soft-Tissue Sarcomas.Int J Mol Sci. 2019 Jan 4;20(1):170. doi: 10.3390/ijms20010170. Int J Mol Sci. 2019. PMID: 30621224 Free PMC article. Review.
-
Alternative Strategies to Inhibit Tumor Vascularization.Int J Mol Sci. 2019 Dec 7;20(24):6180. doi: 10.3390/ijms20246180. Int J Mol Sci. 2019. PMID: 31817884 Free PMC article. Review.
-
ALK1 signaling in development and disease: new paradigms.Cell Mol Life Sci. 2017 Dec;74(24):4539-4560. doi: 10.1007/s00018-017-2636-4. Epub 2017 Sep 4. Cell Mol Life Sci. 2017. PMID: 28871312 Free PMC article. Review.
-
Endoglin in the Spotlight to Treat Cancer.Int J Mol Sci. 2021 Mar 20;22(6):3186. doi: 10.3390/ijms22063186. Int J Mol Sci. 2021. PMID: 33804796 Free PMC article. Review.
-
Endoglin: Beyond the Endothelium.Biomolecules. 2020 Feb 12;10(2):289. doi: 10.3390/biom10020289. Biomolecules. 2020. PMID: 32059544 Free PMC article. Review.
References
-
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90. - PubMed
-
- Duffy A, Greten T. Developing better treatments in hepatocellular carcinoma. Expert Rev Gastroenterol Hepatol. 4:551–60. - PubMed
-
- Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebocontrolled, phase 3 trial. Lancet. 2017;389:56–66. - PubMed
-
- Dallas NA, Samuel S, Xia L, et al. Endoglin (CD105): a marker of tumor vasculature and potential target for therapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:1931–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

