Early AMD-like defects in the RPE and retinal degeneration in aged mice with RPE-specific deletion of Atg5 or Atg7
- PMID: 28465655
- PMCID: PMC5398883
Early AMD-like defects in the RPE and retinal degeneration in aged mice with RPE-specific deletion of Atg5 or Atg7
Abstract
Purpose: To examine the effects of autophagy deficiency induced by RPE-specific deletion of Atg5 or Atg7 in mice as a function of age.
Methods: Conditional knockout mice with a floxed allele of Atg5 or Atg7 were crossed with inducible VMD2-rtTA/Cre transgenic mice. VMD2-directed RPE-specific Cre recombinase expression was induced with doxycycline feeding in the resulting mice. Cre-mediated deletion of floxed Atg5 or Atg7 resulted in RPE-specific inactivation of the Atg5 or Atg7 gene. Plastic and thin retinal sections were analyzed with light and electron microscopy for histological changes. Photoreceptor outer segment (POS) thickness in plastic sections was measured using the Adobe Photoshop CS4 extended ruler tool. Autophagic adaptor p62/SQSTM1 and markers for oxidatively damaged lipids, proteins, and DNA were examined with immunofluorescence staining of cryosections. Fluorescence signals were quantified using Image J software.
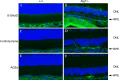
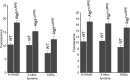
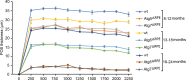
Results: Accumulation of p62/SQSTM1 reflecting autophagy deficiency was observed in the RPE of the Atg5ΔRPE and Atg7ΔRPE mice. 3-nitrotyrosine, advanced glycation end products (AGEs), and 8-hydroxy-2'-deoxyguanosine (8-OHdG), markers for oxidatively damaged proteins and DNA, were also found to accumulate in the RPE of these mice. We observed retinal degeneration in 35% of the Atg5ΔRPE mice and 45% of the Atg7ΔRPE mice at 8 to 24 months old. Degeneration severity and the number of mice with degeneration increased with age. The mean POS thickness of these mice was 25 µm at 8-12 months, 15 µm at 13-18 months, and 3 µm at 19-24 months, compared to 35 µm, 30 µm, and 24 µm in the wild-type mice, respectively. Early age-related macular degeneration (AMD)-like RPE defects were found in all the Atg5ΔRPE and Atg7ΔRPE mice 13 months old or older, including vacuoles, uneven RPE thickness, diminished basal infoldings, RPE hypertrophy/hypotrophy, pigmentary irregularities, and necrosis. The severity of the RPE defects increased with age and in the mice with retinal degeneration. RPE atrophy and choroidal neovascularization (CNV) were occasionally observed in the Atg5ΔRPE and Atg7ΔRPE mice with advanced age.
Conclusions: Autophagy deficiency induced by RPE-specific deletion of Atg5 or Atg7 predisposes but does not necessarily drive the development of AMD-like phenotypes or retinal degeneration.
Figures






Similar articles
-
Di-retinoid-pyridinium-ethanolamine (A2E) Accumulation and the Maintenance of the Visual Cycle Are Independent of Atg7-mediated Autophagy in the Retinal Pigmented Epithelium.J Biol Chem. 2015 Nov 27;290(48):29035-44. doi: 10.1074/jbc.M115.682310. Epub 2015 Oct 14. J Biol Chem. 2015. PMID: 26468292 Free PMC article.
-
Deletion of autophagy inducer RB1CC1 results in degeneration of the retinal pigment epithelium.Autophagy. 2015;11(6):939-53. doi: 10.1080/15548627.2015.1041699. Autophagy. 2015. PMID: 26075877 Free PMC article.
-
Inducible RPE-specific GPX4 knockout causes oxidative stress and retinal degeneration with features of age-related macular degeneration.Exp Eye Res. 2024 Oct;247:110028. doi: 10.1016/j.exer.2024.110028. Epub 2024 Aug 10. Exp Eye Res. 2024. PMID: 39128667
-
Fatty acids and oxidized lipoproteins contribute to autophagy and innate immunity responses upon the degeneration of retinal pigment epithelium and development of age-related macular degeneration.Biochimie. 2019 Apr;159:49-54. doi: 10.1016/j.biochi.2018.07.010. Epub 2018 Jul 18. Biochimie. 2019. PMID: 30031036 Review.
-
Molecular mechanisms and physiological roles of Atg5/Atg7-independent alternative autophagy.Proc Jpn Acad Ser B Phys Biol Sci. 2017;93(6):378-385. doi: 10.2183/pjab.93.023. Proc Jpn Acad Ser B Phys Biol Sci. 2017. PMID: 28603209 Free PMC article. Review.
Cited by
-
Exploring the pathogenesis of age-related macular degeneration: A review of the interplay between retinal pigment epithelium dysfunction and the innate immune system.Front Neurosci. 2022 Nov 3;16:1009599. doi: 10.3389/fnins.2022.1009599. eCollection 2022. Front Neurosci. 2022. PMID: 36408381 Free PMC article. Review.
-
Myelinosome organelles in pathological retinas: ubiquitous presence and dual role in ocular proteostasis maintenance.Neural Regen Res. 2023 May;18(5):1009-1016. doi: 10.4103/1673-5374.355753. Neural Regen Res. 2023. PMID: 36254982 Free PMC article. Review.
-
The endoplasmic reticulum: Homeostasis and crosstalk in retinal health and disease.Prog Retin Eye Res. 2024 Jan;98:101231. doi: 10.1016/j.preteyeres.2023.101231. Epub 2023 Dec 12. Prog Retin Eye Res. 2024. PMID: 38092262 Free PMC article. Review.
-
Autophagy in age-related macular degeneration.Autophagy. 2023 Feb;19(2):388-400. doi: 10.1080/15548627.2022.2069437. Epub 2022 May 1. Autophagy. 2023. PMID: 35468037 Free PMC article. Review.
-
Macroautophagy and aging: The impact of cellular recycling on health and longevity.Mol Aspects Med. 2021 Dec;82:101020. doi: 10.1016/j.mam.2021.101020. Epub 2021 Sep 7. Mol Aspects Med. 2021. PMID: 34507801 Free PMC article. Review.
References
-
- Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–81. - PubMed
-
- Bok D. The retinal pigment epithelium: a versatile partner in vision. J Cell Sci Suppl. 1993;17:189–95. - PubMed
-
- Rubinsztein DC, DiFiglia M, Heintz N, Nixon RA, Qin ZH, Ravikumar B, Stefanis L, Tolkovsky A. Autophagy and its possible roles in nervous system diseases, damage and repair. Autophagy. 2005;1:11–22. - PubMed
-
- Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol. 2007;9:1102–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

