Protein signature characterizing Helicobacter pylori strains of patients with autoimmune atrophic gastritis, duodenal ulcer and gastric cancer
- PMID: 28465717
- PMCID: PMC5408474
- DOI: 10.1186/s13027-017-0133-x
Protein signature characterizing Helicobacter pylori strains of patients with autoimmune atrophic gastritis, duodenal ulcer and gastric cancer
Abstract
Background: Helicobacter pylori (H. pylori) represents a key factor in the etiology of autoimmune atrophic gastritis (AAG), duodenal ulcer (DU) and gastric cancer (GC). The aim of this study was to characterize the differential protein expression of H. pylori isolated from gastric biopsies of patients affected by either AAG, DU or GC.
Methods: The H. pylori strains were isolated from endoscopic biopsies from the stomach of patients with gastric disease. Protein profiles of H. pylori were compared by two-dimensional difference in gel electrophoresis (2D-DIGE) coupled with mass spectrometry (MS) for the identification of significantly different spots (Student t-test, p < 0.05).
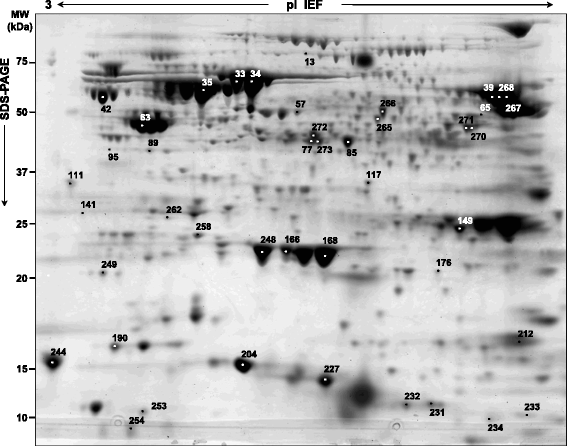
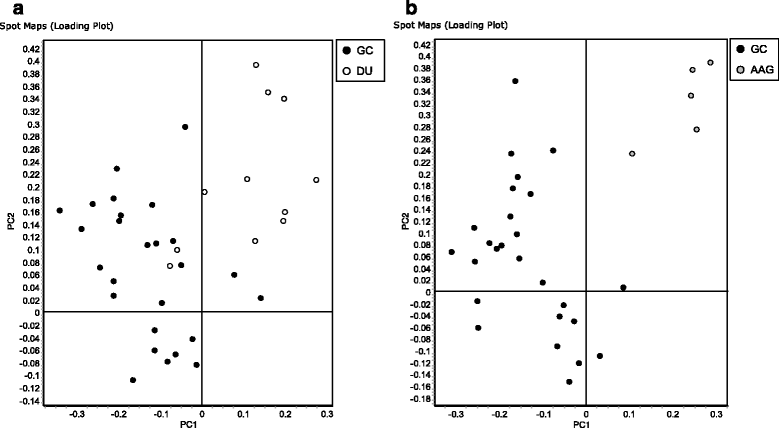
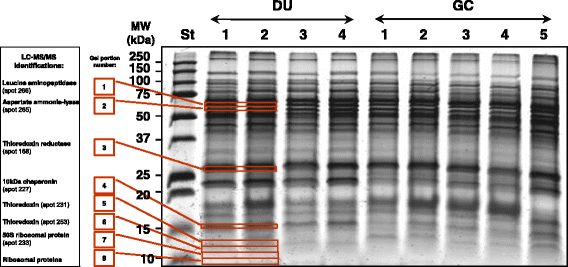
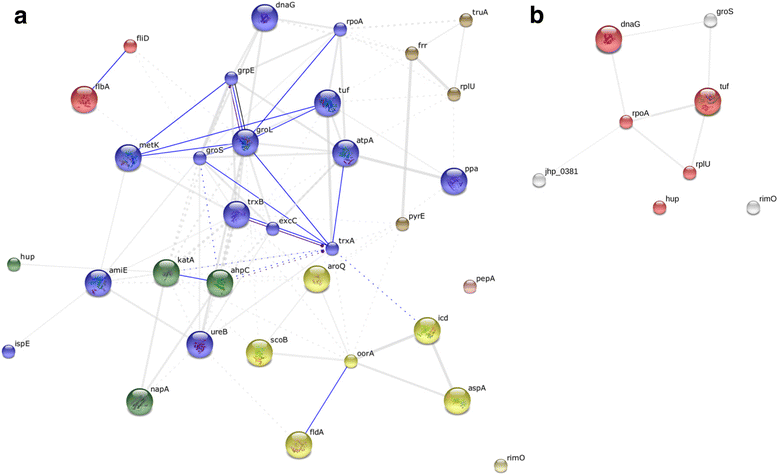
Results: A total of 47 differentially expressed spots were found between H. pylori isolated from patients with either DU or AAG diseases and those isolated from patients with GC (Anova < 0.05, log fold change >1.5). These spots corresponded to 35 unique proteins. The identity of 7 protein spots was validated after one-dimensional electrophoresis and MS/MS analyses of excised gel portions. In H. pylori isolated from DU-patients a significant increase in proteins with antioxidant activity emerged (AroQ, AspA, FldA, Icd, OorA and ScoB), together with a higher content of proteins counteracting the high acid environment (KatA and NapA). In H. pylori isolated from AAG-patients proteins neutralizing hydrogen concentrations through organic substance metabolic processes decreased (GroL, TrxB and Tuf). In addition, a reduction of bacterial motility (FlhA) was found to be associated with AAG-H. pylori isolates. In GC-H. pylori strains it was found an increase in nucleic acid-binding proteins (e.g. DnaG, Tuf, RpoA, RplU) which may be involved in a higher demand of DNA- and protein-related processes.
Conclusion: Our data suggest the presence of specific protein signatures discriminating among H. pylori isolated from either AAG, DU or GC. Changes in protein expression profiles evaluated by DIGE succeeded in deciphering part of the molecular scenarios associated with the different H. pylori-related gastric diseases.
Keywords: Adenocarcinoma; Autoimmune atrophic gastritis; Comparative proteomics; DIGE; Duodenal ulcer; Gastric cancer; Helicobacter pylori.
Figures
References
-
- Naumann M, Sokolova O, Tegtmeyer N, Backert S. Helicobacter pylori: A paradigm pathogen for subverting host cell signal transmission. Trends Microbiol. 2017. pii: S0966-842X(16)30197-4. doi: 10.1016/j.tim.2016.12.004. [Epub ahead of print] - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

