Major and ancillary magnetic resonance features of LI-RADS to assess HCC: an overview and update
- PMID: 28465718
- PMCID: PMC5410075
- DOI: 10.1186/s13027-017-0132-y
Major and ancillary magnetic resonance features of LI-RADS to assess HCC: an overview and update
Abstract
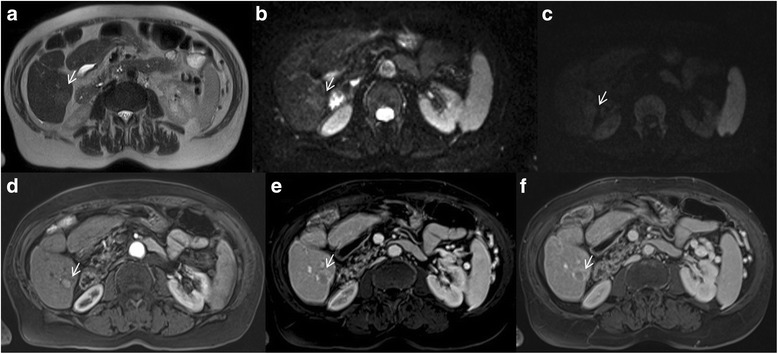
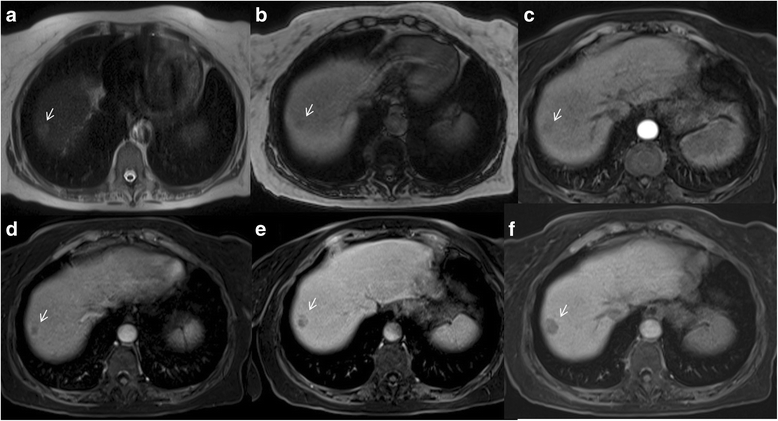
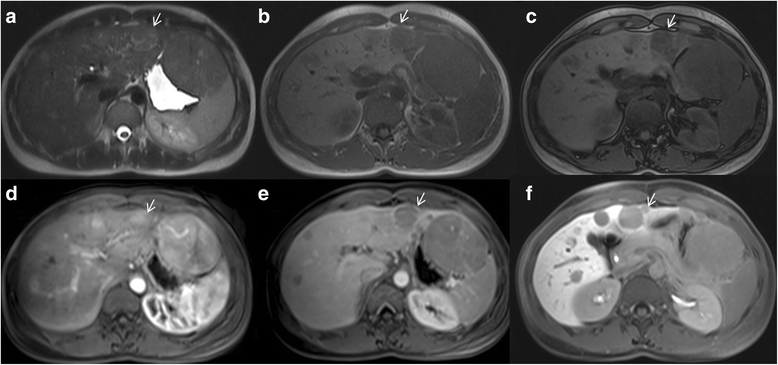
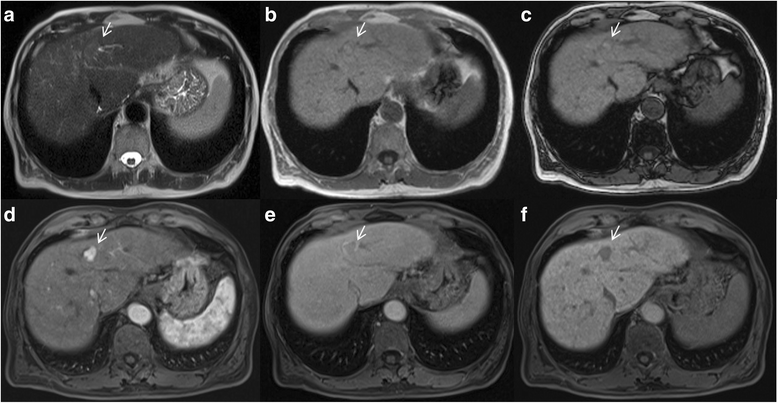
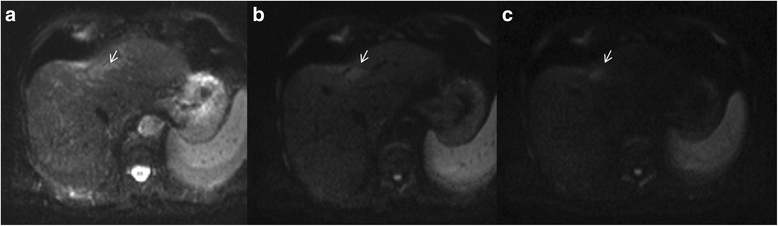
Liver Imaging Reporting and Data System (LI-RADS) is a system for interpreting and reporting of imaging features on multidetector computed tomography (MDCT) and magnetic resonance (MR) studies in patients at risk for hepatocellular carcinoma (HCC). American College of Radiology (ACR) sustained the spread of LI-RADS to homogenizing the interpreting and reporting data of HCC patients. Diagnosis of HCC is due to the presence of major imaging features. Major features are imaging data used to categorize LI-RADS-3, LI-RADS-4, and LI-RADS-5 and include arterial-phase hyperenhancement, tumor diameter, washout appearance, capsule appearance and threshold growth. Ancillary are features that can be used to modify the LI-RADS classification. Ancillary features supporting malignancy (diffusion restriction, moderate T2 hyperintensity, T1 hypointensity on hapatospecifc phase) can be used to upgrade category by one or more categories, but not beyond LI-RADS-4. Our purpose is reporting an overview and update of major and ancillary MR imaging features in assessment of HCC.
Keywords: HCC; LI-RADS; Magnetic resonance imaging.
Figures
Similar articles
-
Critical analysis of major and ancillary features of LI-RADS v2018 in the differentiation of small (≤ 2 cm) hepatocellular carcinoma from dysplastic nodules with gadobenate dimeglumine-enhanced magnetic resonance imaging.Eur Rev Med Pharmacol Sci. 2019 Sep;23(18):7786-7801. doi: 10.26355/eurrev_201909_18988. Eur Rev Med Pharmacol Sci. 2019. PMID: 31599447
-
LI-RADS for MR Imaging Diagnosis of Hepatocellular Carcinoma: Performance of Major and Ancillary Features.Radiology. 2018 Jul;288(1):118-128. doi: 10.1148/radiol.2018171678. Epub 2018 Apr 10. Radiology. 2018. PMID: 29634435
-
LI-RADS for CT diagnosis of hepatocellular carcinoma: performance of major and ancillary features.Abdom Radiol (NY). 2019 Feb;44(2):517-528. doi: 10.1007/s00261-018-1762-2. Abdom Radiol (NY). 2019. PMID: 30167771
-
LI-RADS major features: CT, MRI with extracellular agents, and MRI with hepatobiliary agents.Abdom Radiol (NY). 2018 Jan;43(1):75-81. doi: 10.1007/s00261-017-1291-4. Abdom Radiol (NY). 2018. PMID: 28828680 Review.
-
LI-RADS Version 2018 Ancillary Features at MRI.Radiographics. 2018 Nov-Dec;38(7):1973-2001. doi: 10.1148/rg.2018180052. Epub 2018 Oct 5. Radiographics. 2018. PMID: 30289735 Review.
Cited by
-
Assessment of LI-RADS efficacy in classification of hepatocellular carcinoma and benign liver nodules using DCE-MRI features and machine learning.Eur J Radiol Open. 2023 Oct 31;11:100535. doi: 10.1016/j.ejro.2023.100535. eCollection 2023 Dec. Eur J Radiol Open. 2023. PMID: 37964787 Free PMC article.
-
Preliminary Experience of Liquid Biopsy in Lung Cancer Compared to Conventional Assessment: Light and Shadows.J Pers Med. 2022 Nov 12;12(11):1896. doi: 10.3390/jpm12111896. J Pers Med. 2022. PMID: 36422072 Free PMC article.
-
Scientific Status Quo of Small Renal Lesions: Diagnostic Assessment and Radiomics.J Clin Med. 2024 Jan 18;13(2):547. doi: 10.3390/jcm13020547. J Clin Med. 2024. PMID: 38256682 Free PMC article. Review.
-
Conventional, functional and radiomics assessment for intrahepatic cholangiocarcinoma.Infect Agent Cancer. 2022 Mar 28;17(1):13. doi: 10.1186/s13027-022-00429-z. Infect Agent Cancer. 2022. PMID: 35346300 Free PMC article.
-
Risk Assessment and Cholangiocarcinoma: Diagnostic Management and Artificial Intelligence.Biology (Basel). 2023 Jan 29;12(2):213. doi: 10.3390/biology12020213. Biology (Basel). 2023. PMID: 36829492 Free PMC article. Review.
References
-
- NCCN Clinical Practice Guidelines in Oncology on hepatobiliary cancer. Version 2016. http://www.nccn.org.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

