Biologic Disease-Modifying Antirheumatic Drugs in a National, Privately Insured Population: Utilization, Expenditures, and Price Trends
- PMID: 28465766
- PMCID: PMC5394542
Biologic Disease-Modifying Antirheumatic Drugs in a National, Privately Insured Population: Utilization, Expenditures, and Price Trends
Abstract
Background: Spending on biologic drugs is a significant driver of drug expenditures for payers in private health plans. Biologic disease-modifying antirheumatic drugs (DMARDs) are some of the most effective and costly treatments in a physician's arsenal. Understanding the total annual expenditure, the average cost per prescription, and the impact of cost-sharing is important for drug benefit managers.
Objective: To assess drug utilization, expenditures, out-of-pocket (OOP) cost, and price trends of biologic DMARDs in patients with rheumatoid arthritis (RA) in a large managed care organization.
Methods: We conducted a retrospective database analysis of pharmacy claims data from January 2004 to December 2013 using the Optum Clinformatics Data Mart database, which covers 13.3 million lives. Pharmacy claims for 40,373 patients with RA were identified during the study period. In all, 9 biologic DMARDs approved for the treatment of RA, including infliximab, etanercept, adalimumab, certoizumab, golimumab, tocilizumab, anakinra, abatacept, and rituximab, and 1 nonbiologic oral, small molecule-targeted synthetic drug, tofacitinib, were included in this study. Descriptive statistics were used to analyze the total annual number of prescriptions, the total annual expenditures, the average annual cost per drug (a proxy of drug price), and the average OOP cost (copay plus deductible and coinsurance). All measurements were also stratified by study drugs and by insurance type.
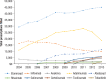
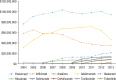
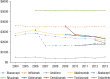
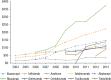
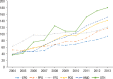
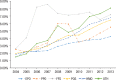
Results: Of the 40,373 patients with RA included in the study, approximately 76% were female (mean age, 55 years at diagnosis). Approximately 77% of the patients were white, and almost 48% lived in the South or Midwest region of the United States. Approximately 62% of patients had a point of service insurance plan. Expenditures on biologic DMARDs increased from $166 million in 2004 to $243 million in 2013, and the number of prescriptions and refills increased from 59,960 in 2004 to 105,295 in 2013. Prescriptions for biologic DMARDs increased more than 20% per patient from 2004 to 2013. The average cost per prescription remained relatively unchanged, at approximately $2300 per prescription, but the OOP expenditures increased from $36 (2.5%) per prescription to $128 (7%) during the study period. The OOP expenditures increased the most in HMO plans and in plans categorized as other (284% and 388%, respectively).
Conclusions: Spending on biologic DMARDs has been primarily driven by an increase in prescribing rates, as the average amount reimbursed per prescription remained relatively unchanged over time, despite a regular annual increase to the average wholesale acquisition cost of 2% to 10%. The OOP burden for patients has increased, but this does not appear to have limited the use of biologic DMARDs. The entrance of new biologic and nonbiologic DMARDs into the market in the past few years is eroding the market share for several established drugs, and may lead to different results, warranting a study of new trends.
Keywords: biologic disease-modifying antirheumatic drugs; drug utilization; expenditures; out-of-pocket cost; price trends; rheumatoid arthritis.
Figures

Similar articles
-
Sociodemographic Determinants of Out-of-Pocket Expenditures for Patients Using Prescription Drugs for Rheumatoid Arthritis.Am Health Drug Benefits. 2017 Feb;10(1):7-15. Am Health Drug Benefits. 2017. PMID: 28465764 Free PMC article.
-
The economic burden of biologic disease-modifying antirheumatic drugs in rheumatoid arthritis patients in the United States.Expert Rev Pharmacoecon Outcomes Res. 2022 Dec;22(8):1231-1241. doi: 10.1080/14737167.2022.2117690. Epub 2022 Sep 6. Expert Rev Pharmacoecon Outcomes Res. 2022. PMID: 36004551
-
Impact of Out-of-Pocket Costs on Prescription Fills Among New Initiators of Biologic Therapies for Rheumatoid Arthritis.J Manag Care Spec Pharm. 2016 Feb;22(2):122-30. doi: 10.18553/jmcp.2016.14261. Epub 2015 Dec 14. J Manag Care Spec Pharm. 2016. PMID: 27015251 Free PMC article.
-
Out-of-Pocket Spending for Retail Prescribed Drugs by Age and Type of Prescription Drug Coverage, 2009 to 2018.2020 Dec. In: Statistical Brief (Medical Expenditure Panel Survey (US)) [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2001–. STATISTICAL BRIEF #532. 2020 Dec. In: Statistical Brief (Medical Expenditure Panel Survey (US)) [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2001–. STATISTICAL BRIEF #532. PMID: 35696500 Free Books & Documents. Review.
-
Integrating Nanotechnological Advancements of Disease-Modifying Anti-Rheumatic Drugs into Rheumatoid Arthritis Management.Pharmaceuticals (Basel). 2024 Feb 14;17(2):248. doi: 10.3390/ph17020248. Pharmaceuticals (Basel). 2024. PMID: 38399463 Free PMC article. Review.
Cited by
-
Patient Preference for Self-Injection Devices in Rheumatoid Arthritis: A Discrete Choice Experiment in China.Patient Prefer Adherence. 2022 Aug 31;16:2387-2398. doi: 10.2147/PPA.S375938. eCollection 2022. Patient Prefer Adherence. 2022. PMID: 36068820 Free PMC article.
-
Cost-Effectiveness of Repository Corticotropin Injection versus Standard of Care for the Treatment of Active Rheumatoid Arthritis.Clinicoecon Outcomes Res. 2021 May 6;13:349-358. doi: 10.2147/CEOR.S304600. eCollection 2021. Clinicoecon Outcomes Res. 2021. PMID: 33986603 Free PMC article.
-
Predicting treatment response to IL6R blockers in rheumatoid arthritis.Rheumatology (Oxford). 2020 Dec 1;59(12):3603-3610. doi: 10.1093/rheumatology/keaa529. Rheumatology (Oxford). 2020. PMID: 32864695 Free PMC article. Review.
-
Illuminating an Invisible Epidemic: A Systemic Review of the Clinical and Economic Benefits of Early Diagnosis and Treatment in Inflammatory Disease and Related Syndromes.J Clin Med. 2019 Apr 11;8(4):493. doi: 10.3390/jcm8040493. J Clin Med. 2019. PMID: 30979036 Free PMC article. Review.
-
Health Care Utilization and Costs in Systemic Therapies for Metastatic Melanoma from 2016 to 2020.Oncologist. 2023 Mar 17;28(3):268-275. doi: 10.1093/oncolo/oyac219. Oncologist. 2023. PMID: 36302223 Free PMC article.
References
-
- Centers for Disease Control and Prevention. Rheumatoid arthritis (RA). Updated July 22, 2016. www.cdc.gov/arthritis/basics/rheumatoid.htm. Accessed June 15, 2016.
-
- Smith HR, Brown A. Rheumatoid arthritis. Medscape. Updated February 2013. http://emedicine.medscape.com/article/331715-overview. Accessed April 4, 2013.
-
- Filipovic I, Walker D, Forster F, Curry AS. Quantifying the economic burden of productivity loss in rheumatoid arthritis. Rheumatology (Oxford). 2011; 50: 1083–1090. - PubMed
-
- Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010; 376: 1094–1108. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
