Comparing Healthcare Costs Associated with Oral and Subcutaneous Methotrexate or Biologic Therapy for Rheumatoid Arthritis in the United States
- PMID: 28465768
- PMCID: PMC5394544
Comparing Healthcare Costs Associated with Oral and Subcutaneous Methotrexate or Biologic Therapy for Rheumatoid Arthritis in the United States
Abstract
Background: Methotrexate (MTX) is the primary disease-modifying antirheumatic drug used for the treatment of rheumatoid arthritis (RA). Optimizing the use of oral and subcutaneous MTX may delay the use of expensive biologic therapies; the effect of such a delay on overall medical costs is currently unknown.
Objective: To compare the 5-year healthcare costs of treatment pathways for patients with RA who initiate oral MTX in the United States.
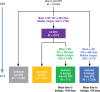
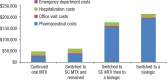
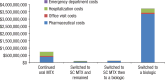
Methods: We identified patients with RA in the Symphony Health Solutions database (Integrated Dataverse) who initiated treatment with oral MTX in 2009 and had RA-related claims for each year through 2014. We then grouped the patients into 4 treatment cohorts, including those who (1) continued to use oral MTX, (2) switched to subcutaneous MTX, (3) switched to subcutaneous MTX and then added or switched to a biologic therapy, and (4) added or switched to a biologic therapy. The costs (in 2015 US dollars) for pharmaceuticals, office visits, hospitalizations, and emergency department visits were estimated for each cohort.
Results: Of the total 35,640 patients in this study, 15,599 patients continued to use oral MTX, with an average cost of $47,464 per patient in the full study period; 1802 patients switched to subcutaneous MTX, with an average per-patient cost of $59,058; 711 patients switched to subcutaneous MTX and then added or switched to a biologic agent, with an average per-patient cost of $175,391 and a mean time to a biologic use of 1184 days; and 17,528 patients added or switched to a biologic from oral MTX, with an average per-patient cost of $212,595 and a mean time to a biologic use of 478 days. Biologic treatments were responsible for the cost differences between the cohorts; the nondrug costs were similar across the groups.
Conclusion: Our findings that patients who switched to subcutaneous MTX incurred lower costs than patients who only used oral MTX before using biologics may provide useful information for patients and providers who are choosing between continued MTX use and adding or switching to a biologic based on treatment guidelines.
Keywords: MTX; biologic therapy; healthcare cost; oral methotrexate; rheumatoid arthritis; subcutaneous methotrexate; treatment pathways.
Figures
Similar articles
-
Use of oral and subcutaneous methotrexate in rheumatoid arthritis patients in the United States.Arthritis Care Res (Hoboken). 2014 Nov;66(11):1604-11. doi: 10.1002/acr.22383. Arthritis Care Res (Hoboken). 2014. PMID: 24942466
-
Cost-minimisation analysis of subcutaneous methotrexate versus biologic therapy for the treatment of patients with rheumatoid arthritis who have had an insufficient response or intolerance to oral methotrexate.Clin Rheumatol. 2013 Nov;32(11):1605-12. doi: 10.1007/s10067-013-2318-z. Epub 2013 Jul 9. Clin Rheumatol. 2013. PMID: 23835658
-
Underuse of Methotrexate in the Treatment of Rheumatoid Arthritis: A National Analysis of Prescribing Practices in the US.Arthritis Care Res (Hoboken). 2017 Jun;69(6):794-800. doi: 10.1002/acr.23152. Epub 2017 Apr 24. Arthritis Care Res (Hoboken). 2017. PMID: 27863180
-
Methotrexate in rheumatoid arthritis: optimizing therapy among different formulations. Current and emerging paradigms.Clin Ther. 2014 Mar 1;36(3):427-35. doi: 10.1016/j.clinthera.2014.01.014. Epub 2014 Mar 5. Clin Ther. 2014. PMID: 24612941 Review.
-
Tofacitinib for Treating Rheumatoid Arthritis After the Failure of Disease-Modifying Anti-rheumatic Drugs: An Evidence Review Group Perspective of a NICE Single Technology Appraisal.Pharmacoeconomics. 2018 Sep;36(9):1063-1072. doi: 10.1007/s40273-018-0639-0. Pharmacoeconomics. 2018. PMID: 29546668 Review.
Cited by
-
Methotrexate Shortages and Their Impact on Chronic Inflammatory Rheumatic Disease Management: Results From a National Survey of Moroccan Rheumatologists.Cureus. 2025 Apr 20;17(4):e82640. doi: 10.7759/cureus.82640. eCollection 2025 Apr. Cureus. 2025. PMID: 40395257 Free PMC article.
-
Potential Benefits of the Self-Administration of Subcutaneous Methotrexate with Autoinjector Devices for Patients: A Review.Drug Healthc Patient Saf. 2021 Mar 29;13:81-94. doi: 10.2147/DHPS.S290771. eCollection 2021. Drug Healthc Patient Saf. 2021. PMID: 33824602 Free PMC article. Review.
-
Real-World Healthcare Resource Utilization and Cost Burden Assessment for Adults With Generalized Myasthenia Gravis in the United States.Front Neurol. 2022 Jan 18;12:809999. doi: 10.3389/fneur.2021.809999. eCollection 2021. Front Neurol. 2022. PMID: 35115997 Free PMC article.
-
Naloxone and Buprenorphine Prescribing Following US Emergency Department Visits for Suspected Opioid Overdose: August 2019 to April 2021.Ann Emerg Med. 2022 Mar;79(3):225-236. doi: 10.1016/j.annemergmed.2021.10.005. Epub 2021 Nov 19. Ann Emerg Med. 2022. PMID: 34802772 Free PMC article.
-
A strategy to identify event specific hospitalizations in large health claims databases.BMC Health Serv Res. 2022 May 26;22(1):705. doi: 10.1186/s12913-022-08107-x. BMC Health Serv Res. 2022. PMID: 35619126 Free PMC article.
References
-
- Helmick CG, Felson DT, Lawrence RC, et al; for the National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: part I. Arthritis Rheum. 2008; 58: 15–25. - PubMed
-
- Matcham F, Scott IC, Rayner L, et al. The impact of rheumatoid arthritis on quality-of-life assessed using the SF-36: a systematic review and meta-analysis. Semin Arthritis Rheum. 2014; 44: 123–130. - PubMed
-
- Kawatkar AA, Jacobsen SJ, Levy GD, et al. Direct medical expenditure associated with rheumatoid arthritis in a nationally representative sample from the Medical Expenditure Panel Survey. Arthritis Care Res (Hoboken). 2012; 64: 1649–1656. - PubMed
-
- Singh JA, Saag KG, Bridges SL, Jr, et al. 2015. American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016; 68: 1–26. - PubMed
LinkOut - more resources
Full Text Sources