Comparing Healthcare Costs Associated with Oral and Subcutaneous Methotrexate or Biologic Therapy for Rheumatoid Arthritis in the United States
- PMID: 28465768
- PMCID: PMC5394544
Comparing Healthcare Costs Associated with Oral and Subcutaneous Methotrexate or Biologic Therapy for Rheumatoid Arthritis in the United States
Abstract
Background: Methotrexate (MTX) is the primary disease-modifying antirheumatic drug used for the treatment of rheumatoid arthritis (RA). Optimizing the use of oral and subcutaneous MTX may delay the use of expensive biologic therapies; the effect of such a delay on overall medical costs is currently unknown.
Objective: To compare the 5-year healthcare costs of treatment pathways for patients with RA who initiate oral MTX in the United States.
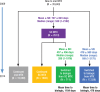
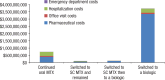
Methods: We identified patients with RA in the Symphony Health Solutions database (Integrated Dataverse) who initiated treatment with oral MTX in 2009 and had RA-related claims for each year through 2014. We then grouped the patients into 4 treatment cohorts, including those who (1) continued to use oral MTX, (2) switched to subcutaneous MTX, (3) switched to subcutaneous MTX and then added or switched to a biologic therapy, and (4) added or switched to a biologic therapy. The costs (in 2015 US dollars) for pharmaceuticals, office visits, hospitalizations, and emergency department visits were estimated for each cohort.
Results: Of the total 35,640 patients in this study, 15,599 patients continued to use oral MTX, with an average cost of $47,464 per patient in the full study period; 1802 patients switched to subcutaneous MTX, with an average per-patient cost of $59,058; 711 patients switched to subcutaneous MTX and then added or switched to a biologic agent, with an average per-patient cost of $175,391 and a mean time to a biologic use of 1184 days; and 17,528 patients added or switched to a biologic from oral MTX, with an average per-patient cost of $212,595 and a mean time to a biologic use of 478 days. Biologic treatments were responsible for the cost differences between the cohorts; the nondrug costs were similar across the groups.
Conclusion: Our findings that patients who switched to subcutaneous MTX incurred lower costs than patients who only used oral MTX before using biologics may provide useful information for patients and providers who are choosing between continued MTX use and adding or switching to a biologic based on treatment guidelines.
Keywords: MTX; biologic therapy; healthcare cost; oral methotrexate; rheumatoid arthritis; subcutaneous methotrexate; treatment pathways.
Figures
References
-
- Helmick CG, Felson DT, Lawrence RC, et al; for the National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: part I. Arthritis Rheum. 2008; 58: 15–25. - PubMed
-
- Matcham F, Scott IC, Rayner L, et al. The impact of rheumatoid arthritis on quality-of-life assessed using the SF-36: a systematic review and meta-analysis. Semin Arthritis Rheum. 2014; 44: 123–130. - PubMed
-
- Kawatkar AA, Jacobsen SJ, Levy GD, et al. Direct medical expenditure associated with rheumatoid arthritis in a nationally representative sample from the Medical Expenditure Panel Survey. Arthritis Care Res (Hoboken). 2012; 64: 1649–1656. - PubMed
-
- Singh JA, Saag KG, Bridges SL, Jr, et al. 2015. American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016; 68: 1–26. - PubMed
LinkOut - more resources
Full Text Sources