Appropriate endpoints for evaluation of new antibiotic therapies for severe infections: a perspective from COMBACTE's STAT-Net
- PMID: 28466147
- PMCID: PMC5487537
- DOI: 10.1007/s00134-017-4802-4
Appropriate endpoints for evaluation of new antibiotic therapies for severe infections: a perspective from COMBACTE's STAT-Net
Abstract
Purpose: In this era of rising antimicrobial resistance, slowly refilling antibiotic development pipelines, and an aging population, we need to ensure that randomized clinical trials (RCTs) determine the added benefit of new antibiotic agents effectively and in a valid way, especially for severely ill patients. Unfortunately, universally accepted endpoints for the evaluation of new drugs in severe infections are lacking.
Methods: We review and discuss the current practices and challenges regarding endpoints in RCTs in this field and propose novel approaches.
Results: Usual endpoints actually recommended for drug development suffer from important flaws. Mortality requires large sample size and only partly related to the infectious process. Clinical cure rate is highly subjective in critically ill patients where symptoms may be related to other intercurrent events. Currently, composite endpoints, hierarchical nested designs, and competing risks analysis seem to be the most promising new tools for designing and analyzing clinical trials in this area, although they require further validation.
Conclusion: Regulatory authorities, pharmaceutical companies, and clinicians need to agree on the most appropriate clinical endpoints for severe infections to ensure efficient approval of new, effective antibiotic agents.
Keywords: Antibiotic therapy; Consensus; Endpoints; Randomized clinical trials; Severe infections.
Conflict of interest statement
Conflicts of interests
JFT has received speaker fees from Astellas, Gilead, Novartis, Merck, and Pfizer, and is Advisory Board member for 3M, Bayer, Abbott, and Merck. SH has received consulting fees from GSK, Janssen, Abbott, and Novartis, as well as research support from Pfizer.
Funding
For all authors, the research leading to this manuscript has received support from the Innovative Medicines Initiative Joint Undertaking under Grant agreement No. 115523 [Combatting Bacterial Resistance in Europe projects (COMBACTE)], resources of which are composed of financial contribution from the European Union’s 7th Framework Programme (FP7/2007 ± 2013) and the European Federation of Pharmaceutical Industries and Associations (EFPIA) companies’ in-kind contribution. MW has also received funding from the German Research Foundation (Deutsche Forschungsgemeinschaft) under Grant No. WO 1746/1-2. DW belongs to an EFPIA member company in the IMI JU and costs related to his part in the research was carried by the respective company as an in-kind contribution under the IMI JU scheme. The funders had no role in data collection and analysis, decision to publish, or preparation of the manuscript.
Figures

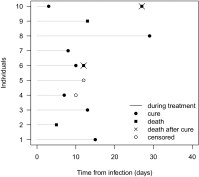
References
-
- Talbot GH, Powers JH, Fleming TR, Siuciak JA, Bradley J, Boucher H. Progress on developing endpoints for registrational clinical trials of community-acquired bacterial pneumonia and acute bacterial skin and skin structure infections: update from the Biomarkers Consortium of the Foundation for the National Institutes of Health. Clin Infect Dis. 2012;55:1114–1121. doi: 10.1093/cid/cis566. - DOI - PMC - PubMed
-
- Harris PN, McNamara JF, Lye DC, Davis JS, Bernard L, Cheng AC, Doi Y, Fowler VG, Jr., Kaye KS, Leibovici L, Lipman J, Llewelyn MJ, Munoz-Price S, Paul M, Peleg AY, Rodriguez-Bano J, Rogers BA, Seifert H, Thamlikitkul V, Thwaites G, Tong SY, Turnidge J, Utili R, Webb SA, Paterson DL. Proposed primary endpoints for use in clinical trials that compare treatment options for bloodstream infection in adults: a consensus definition. Clin Microbiol Infect. 2016 - PubMed
-
- U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research . Guidance for industry hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia: developing drugs for treatment. Silver Spring: Center for Drug Evaluation and Research; 2014.
-
- Garrouste Orgeas M, Timsit JF, Soufir L, Tafflet M, Adrie C, Philippart F, Zahar JR, Clec’h C, Goldran-Toledano D, Jamali S, Dumenil AS, Azoulay E, Carlet J. Impact of adverse events on outcomes in intensive care unit patients. Crit Care Med. 2008;36:2041–2047. doi: 10.1097/CCM.0b013e31817b879c. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

