Cortical Representation of Pain and Touch: Evidence from Combined Functional Neuroimaging and Electrophysiology in Non-human Primates
- PMID: 28466257
- PMCID: PMC5799120
- DOI: 10.1007/s12264-017-0133-2
Cortical Representation of Pain and Touch: Evidence from Combined Functional Neuroimaging and Electrophysiology in Non-human Primates
Abstract
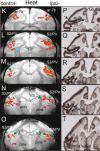
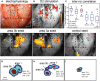
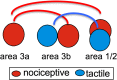
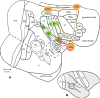
Human functional MRI studies in acute and various chronic pain conditions have revolutionized how we view pain, and have led to a new theory that complex multi-dimensional pain experience (sensory-discriminative, affective/motivational, and cognitive) is represented by concurrent activity in widely-distributed brain regions (termed a network or pain matrix). Despite these breakthrough discoveries, the specific functions proposed for these regions remain elusive, because detailed electrophysiological characterizations of these regions in the primate brain are lacking. To fill in this knowledge gap, we have studied the cortical areas around the central and lateral sulci of the non-human primate brain with combined submillimeter resolution functional imaging (optical imaging and fMRI) and intracranial electrophysiological recording. In this mini-review, I summarize and present data showing that the cortical circuitry engaged in nociceptive processing is much more complex than previously recognized. Electrophysiological evidence supports the engagement of a distinct nociceptive-processing network within SI (i.e., areas 3a, 3b, 1 and 2), SII, and other areas along the lateral sulcus. Deafferentation caused by spinal cord injury profoundly alters the relationships between fMRI and electrophysiological signals. This finding has significant implications for using fMRI to study chronic pain conditions involving deafferentation in humans.
Keywords: Cortex; Functional MRI; Functional connectivity; Nociception; Non-human primate.
Figures


Similar articles
-
Discrete Modules and Mesoscale Functional Circuits for Thermal Nociception within Primate S1 Cortex.J Neurosci. 2018 Feb 14;38(7):1774-1787. doi: 10.1523/JNEUROSCI.2795-17.2017. Epub 2018 Jan 15. J Neurosci. 2018. PMID: 29335352 Free PMC article.
-
A thermal nociceptive patch in the S2 cortex of nonhuman primates: a combined functional magnetic resonance imaging and electrophysiology study.Pain. 2021 Nov 1;162(11):2705-2716. doi: 10.1097/j.pain.0000000000002247. Pain. 2021. PMID: 33945242 Free PMC article.
-
Optical imaging of nociception in primary somatosensory cortex of non-human primates.Sheng Li Xue Bao. 2008 Oct 25;60(5):664-8. Sheng Li Xue Bao. 2008. PMID: 18958375
-
The cortical representation of pain.Pain. 1999 Feb;79(2-3):105-11. doi: 10.1016/s0304-3959(98)00184-5. Pain. 1999. PMID: 10068155 Review.
-
Biophysical and neural basis of resting state functional connectivity: Evidence from non-human primates.Magn Reson Imaging. 2017 Jun;39:71-81. doi: 10.1016/j.mri.2017.01.020. Epub 2017 Feb 2. Magn Reson Imaging. 2017. PMID: 28161319 Free PMC article. Review.
Cited by
-
The Hedonic Experience Associated with a Gentle Touch Is Preserved in Women with Fibromyalgia.J Clin Med. 2024 Sep 23;13(18):5649. doi: 10.3390/jcm13185649. J Clin Med. 2024. PMID: 39337136 Free PMC article.
-
Differential dose responses of transcranial focused ultrasound at brain regions indicate causal interactions.Brain Stimul. 2022 Nov-Dec;15(6):1552-1564. doi: 10.1016/j.brs.2022.12.003. Epub 2022 Dec 7. Brain Stimul. 2022. PMID: 36496128 Free PMC article.
-
Gaps in Understanding Mechanism and Lack of Treatments: Potential Use of a Nonhuman Primate Model of Oxaliplatin-Induced Neuropathic Pain.Pain Res Manag. 2018 May 2;2018:1630709. doi: 10.1155/2018/1630709. eCollection 2018. Pain Res Manag. 2018. PMID: 29854035 Free PMC article. Review.
-
Distinct functional cerebral hypersensitivity networks during incisional and inflammatory pain in rats.Curr Res Neurobiol. 2024 Nov 23;8:100142. doi: 10.1016/j.crneur.2024.100142. eCollection 2025 Jun. Curr Res Neurobiol. 2024. PMID: 39810939 Free PMC article.
-
The Modulatory Effect of Motor Cortex Astrocytes on Diabetic Neuropathic Pain.J Neurosci. 2021 Jun 16;41(24):5287-5302. doi: 10.1523/JNEUROSCI.2566-20.2021. Epub 2021 Mar 22. J Neurosci. 2021. PMID: 33753547 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

