Current status and perspectives of chimeric antigen receptor modified T cells for cancer treatment
- PMID: 28466386
- PMCID: PMC5712290
- DOI: 10.1007/s13238-017-0400-z
Current status and perspectives of chimeric antigen receptor modified T cells for cancer treatment
Abstract
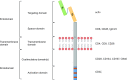
Chimeric antigen receptor (CAR) is a recombinant immunoreceptor combining an antibody-derived targeting fragment with signaling domains capable of activating cells, which endows T cells with the ability to recognize tumor-associated surface antigens independent of the expression of major histocompatibility complex (MHC) molecules. Recent early-phase clinical trials of CAR-modified T (CAR-T) cells for relapsed or refractory B cell malignancies have demonstrated promising results (that is, anti-CD19 CAR-T in B cell acute lymphoblastic leukemia (B-ALL)). Given this success, broadening the clinical experience of CAR-T cell therapy beyond hematological malignancies has been actively investigated. Here we discuss the basic design of CAR and review the clinical results from the studies of CAR-T cells in B cell leukemia and lymphoma, and several solid tumors. We additionally discuss the major challenges in the further development and strategies for increasing anti-tumor activity and safety, as well as for successful commercial translation.
Keywords: CAR-T; adoptive cell therapy; cancer treatment; chimeric antigen receptor; engineered T cells.
Figures

References
-
- Ahmed N, Brawley V, Diouf O, Anderson P, Hicks J, Wang L, Dotti G, Wels W, Liu H, Gee A, et al. Abstract 3500: T cells redirected against HER2 for the adoptive immunotherapy for HER2-positive osteosarcoma. Cancer Res. 2014;72(8 Supplement):3500. doi: 10.1158/1538-7445.AM2012-3500. - DOI
-
- Ahmed N, Brawley VS, Hegde M, Robertson C, Ghazi A, Gerken C, Liu E, Dakhova O, Ashoori A, Corder A, et al. Human epidermal growth factor receptor 2 (HER2)—specific chimeric antigen receptor-modified T cells for the immunotherapy of HER2-positive sarcoma. J Clin Oncol. 2015;33(15):1688–1696. doi: 10.1200/JCO.2014.58.0225. - DOI - PMC - PubMed
-
- Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, Abrams J, Sznol M, Parkinson D, Hawkins M, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17(7):2105–2116. doi: 10.1200/JCO.1999.17.7.2105. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

