Identification and Management of Statin-Associated Symptoms in Clinical Practice: Extension of a Clinician Survey to 12 Further Countries
- PMID: 28466399
- PMCID: PMC5427112
- DOI: 10.1007/s10557-017-6727-0
Identification and Management of Statin-Associated Symptoms in Clinical Practice: Extension of a Clinician Survey to 12 Further Countries
Abstract
Purpose: Statins are the first-choice pharmacological treatment for patients with hypercholesterolemia and at risk for cardiovascular disease; however, a minority of patients experience statin-associated symptoms (SAS) and are considered to have reduced statin tolerance. The objective of this study was to establish how patients with SAS are identified and managed in clinical practice in Austria, Belgium, Colombia, Croatia, the Czech Republic, Denmark, Portugal, Switzerland, Russia, Saudi Arabia, Turkey, and the United Arab Emirates.
Methods: A cross-sectional survey was conducted (2015-2016) among clinicians (n = 60 per country; Croatia: n = 30) who are specialized/experienced in the treatment of hypercholesterolemia. Participants were asked about their experience of patients presenting with potential SAS and how such patients were identified and treated.
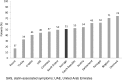
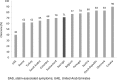
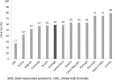
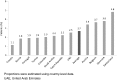
Results: Muscle-related symptoms were the most common presentation of potential SAS (average: 51%; range across countries [RAC] 17-74%); other signs/symptoms included persistent elevation in transaminases. To establish whether symptoms are due to statins, clinicians required rechallenge after discontinuation of statin treatment (average: 77%; RAC 40-90%); other requirements included trying at least one alternative statin. Clinicians reported that half of high-risk patients with confirmed SAS receive a lower-dose statin (average: 53%; RAC 43-72%), and that most receive another non-statin lipid-lowering therapy with or without a concomitant statin (average: 65%; RAC 52-83%).
Conclusions: The specialists and GPs surveyed use stringent criteria to establish causality between statin use and signs or symptoms, and persevere with statin treatment where possible.
Keywords: Clinical practice; Hypercholesterolemia; Reduced statin tolerance; Statin-associated muscle symptoms; Statin-associated symptoms.
Conflict of interest statement
Funding
This survey was sponsored by Amgen Inc.
Conflict of Interest
SRG is an Amgen Inc. employee and stockholder. LC and RD were employees and stockholders of Amgen Inc. during the time of the study. JM and HW are PRMA Consulting employees. RR has received research funding from Amgen, Astra Zeneca, Medicines Company, Regeneron and Sanofi Aventis; he serves as a consultant/advisory board member to Akcea, Amgen, Astra Zeneca, CVS Caremark, Eli Lilly, Regeneron and Sanofi Aventis and has received honoraria from Kowa, and royalties from UpToDate, Inc. GKH, or his institution, has received honoraria for consultancy and advisory boards and received research support and/or conduct of clinical trials from Amgen, Aegerion, Pfizer, Astra Zeneca, Sanofi, Regeneron, Kowa, Ionis pharmaceuticals, Synageva and Cerenis. AC has received honoraria, lecture fees, or research grants from SigmaTau, Manarini, Kowa, Recordati, Eli Lilly, Pfizer, Sanofi, Mediolanum, Merck, Aegerion, Amgen, Astra Zeneca, Genzyme and Bayer. ES has received research support and/or conduct of clinical trials from Amgen, Sanofi and Pfizer, and has served as a consultant for Amgen, Sanofi, Merck, Novartis, Cerenuis and Isis. He has also participated in a Speakers Bureau for Medcon Europe and held non-renumerative positions of influence for Ionis and Chiesi. PO has served as an advisory board member for Amgen.
Research Involving Human Participants and/or Animals
The study was approved by the Human Research Ethics Committee of the University of Technology, Sydney (reference number: PRMA3407_2015_09).
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Figures
References
-
- Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. European guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. Atherosclerosis. 2016;252:207–274. doi: 10.1016/j.atherosclerosis.2016.05.037. - DOI - PubMed
-
- Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, et al. ESC/EAS guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) Atherosclerosis. 2016;253:281–344. doi: 10.1016/j.atherosclerosis.2016.08.018. - DOI - PubMed
-
- Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267–1278. doi: 10.1016/S0140-6736(05)67394-1. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

