The comparative immunology of wild and laboratory mice, Mus musculus domesticus
- PMID: 28466840
- PMCID: PMC5418598
- DOI: 10.1038/ncomms14811
The comparative immunology of wild and laboratory mice, Mus musculus domesticus
Abstract
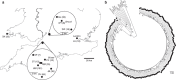
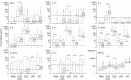
The laboratory mouse is the workhorse of immunology, used as a model of mammalian immune function, but how well immune responses of laboratory mice reflect those of free-living animals is unknown. Here we comprehensively characterize serological, cellular and functional immune parameters of wild mice and compare them with laboratory mice, finding that wild mouse cellular immune systems are, comparatively, in a highly activated (primed) state. Associations between immune parameters and infection suggest that high level pathogen exposure drives this activation. Moreover, wild mice have a population of highly activated myeloid cells not present in laboratory mice. By contrast, in vitro cytokine responses to pathogen-associated ligands are generally lower in cells from wild mice, probably reflecting the importance of maintaining immune homeostasis in the face of intense antigenic challenge in the wild. These data provide a comprehensive basis for validating (or not) laboratory mice as a useful and relevant immunological model system.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





References
-
- Yang H., Bell T. A., Churchill G. A. & Pardo-Manuel de Villena F. On the subspecific origin of the laboratory mouse. Nat. Genet. 39, 1100–1107 (2007). - PubMed
-
- Wade C. M. et al.. The mosaic structure of variation in the laboratory mouse genome. Nature 420, 574–578 (2002). - PubMed
-
- Sellers R. S., Clifford C. B., Treuting P. M. & Brayton C. Immunological variation between inbred laboratory mouse strains: points to consider in phenotyping genetically immunomodified mice. Vet. Pathol. 49, 32–43 (2012). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

