The careg element reveals a common regulation of regeneration in the zebrafish myocardium and fin
- PMID: 28466843
- PMCID: PMC5418624
- DOI: 10.1038/ncomms15151
The careg element reveals a common regulation of regeneration in the zebrafish myocardium and fin
Abstract
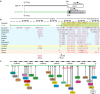
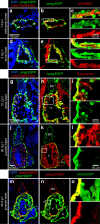
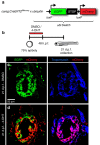
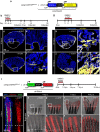
The existence of common mechanisms regulating organ regeneration is an intriguing concept. Here we report on a regulatory element that is transiently activated during heart and fin regeneration in zebrafish. This element contains a ctgfa upstream sequence, called careg, which is induced by TGFβ/Activin-β signalling in the peri-injury zone of the myocardium and the fin mesenchyme. In addition, this reporter demarcates a primordial cardiac layer and intraray osteoblasts. Using genetic fate mapping, we show the regenerative competence of careg-expressing cells. The analysis of the heart reveals that the primordial cardiac layer is incompletely restored after cryoinjury, whereas trabecular and cortical cardiomyocytes contribute to myocardial regrowth. In regenerating fins, the activated mesenchyme of the stump gives rise to the blastema. Our findings provide evidence of a common regenerative programme in cardiomyocytes and mesenchyme that opens the possibility to further explore conserved mechanisms of the cellular plasticity in diverse vertebrate organs.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





Similar articles
-
Modulation of tissue repair by regeneration enhancer elements.Nature. 2016 Apr 14;532(7598):201-6. doi: 10.1038/nature17644. Epub 2016 Apr 6. Nature. 2016. PMID: 27049946 Free PMC article.
-
A dual epimorphic and compensatory mode of heart regeneration in zebrafish.Dev Biol. 2015 Mar 1;399(1):27-40. doi: 10.1016/j.ydbio.2014.12.002. Epub 2014 Dec 31. Dev Biol. 2015. PMID: 25557620
-
Activin-betaA signaling is required for zebrafish fin regeneration.Curr Biol. 2007 Aug 21;17(16):1390-5. doi: 10.1016/j.cub.2007.07.019. Epub 2007 Aug 2. Curr Biol. 2007. PMID: 17683938
-
Zebrafish fin and heart: what's special about regeneration?Curr Opin Genet Dev. 2016 Oct;40:48-56. doi: 10.1016/j.gde.2016.05.011. Epub 2016 Jun 25. Curr Opin Genet Dev. 2016. PMID: 27351724 Review.
-
Linking wound response and inflammation to regeneration in the zebrafish larval fin.Int J Dev Biol. 2018;62(6-7-8):473-477. doi: 10.1387/ijdb.170331hr. Int J Dev Biol. 2018. PMID: 29938759 Review.
Cited by
-
Hemodynamic Forces Regulate Cardiac Regeneration-Responsive Enhancer Activity during Ventricle Regeneration.Int J Mol Sci. 2021 Apr 11;22(8):3945. doi: 10.3390/ijms22083945. Int J Mol Sci. 2021. PMID: 33920448 Free PMC article.
-
Turning gray selenium and sublimed sulfur into a nanocomposite to accelerate tissue regeneration by isothermal recrystallization.J Nanobiotechnology. 2023 Feb 21;21(1):57. doi: 10.1186/s12951-023-01796-4. J Nanobiotechnology. 2023. PMID: 36803772 Free PMC article.
-
Distinct features of the regenerating heart uncovered through comparative single-cell profiling.Biol Open. 2024 Apr 15;13(4):bio060156. doi: 10.1242/bio.060156. Epub 2024 Apr 5. Biol Open. 2024. PMID: 38526188 Free PMC article.
-
Cardiac enhancers: Gateway to the regulatory mechanisms of heart regeneration.Semin Cell Dev Biol. 2025 Jun;170:103610. doi: 10.1016/j.semcdb.2025.103610. Epub 2025 Apr 10. Semin Cell Dev Biol. 2025. PMID: 40215762 Review.
-
A Systematic Exposition of Methods used for Quantification of Heart Regeneration after Apex Resection in Zebrafish.Cells. 2020 Feb 26;9(3):548. doi: 10.3390/cells9030548. Cells. 2020. PMID: 32111059 Free PMC article.
References
-
- Sehring I. M., Jahn C. & Weidinger G. Zebrafish fin and heart: what's special about regeneration? Curr. Opin. Genet. Dev. 40, 48–56 (2016). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

