Stem cell proliferation patterns as an alternative for in vivo prediction and discrimination of carcinogenic compounds
- PMID: 28466856
- PMCID: PMC5413882
- DOI: 10.1038/srep45616
Stem cell proliferation patterns as an alternative for in vivo prediction and discrimination of carcinogenic compounds
Abstract
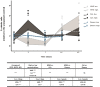
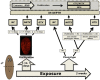
One of the major challenges in the development of alternative carcinogenicity assays is the prediction of non-genotoxic carcinogens. The variety of non-genotoxic cancer pathways complicates the search for reliable parameters expressing their carcinogenicity. As non-genotoxic and genotoxic carcinogens have different cancer risks, the objective of this study was to develop a concept for an in vivo test, based on flatworm stem cell dynamics, to detect and classify carcinogenic compounds. Our methodology entails an exposure to carcinogenic compounds during the animal's regeneration process, which revealed differences in proliferative responses between non-genotoxic and genotoxic carcinogens during the initial stages of the regeneration process. A proof of concept was obtained after an extensive study of proliferation dynamics of a genotoxic and a non-genotoxic compound. A pilot validation with a limited set of compounds showed that the proposed concept not only enabled a simple prediction of genotoxic and non-genotoxic carcinogens, but also had the power to discriminate between both. We further optimized this discrimination by combining stem cell proliferation responses with a phenotypic screening and by using specific knockdowns. In the future, more compounds will be tested to further validate and prove this concept.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Similar articles
-
Epigenetic events during the process of cell transformation induced by carcinogens (review).Oncol Rep. 1999 Jul-Aug;6(4):925-32. doi: 10.3892/or.6.4.925. Oncol Rep. 1999. PMID: 10373683 Review.
-
Guidelines for the evaluation of chemicals for carcinogenicity. Committee on Carcinogenicity of Chemicals in Food, Consumer Products and the Environment.Rep Health Soc Subj (Lond). 1991;42:1-80. Rep Health Soc Subj (Lond). 1991. PMID: 1763238
-
Comparison of hepatocarcinogen-induced gene expression profiles in conventional primary rat hepatocytes with in vivo rat liver.Arch Toxicol. 2012 Sep;86(9):1399-411. doi: 10.1007/s00204-012-0847-x. Epub 2012 Apr 8. Arch Toxicol. 2012. PMID: 22484513
-
An integrative test strategy for cancer hazard identification.Crit Rev Toxicol. 2016 Aug;46(7):615-39. doi: 10.3109/10408444.2016.1171294. Epub 2016 May 3. Crit Rev Toxicol. 2016. PMID: 27142259
-
Neonatal mouse model: review of methods and results.Toxicol Pathol. 2001;29 Suppl:128-37. doi: 10.1080/019262301753178537. Toxicol Pathol. 2001. PMID: 11695548 Review.
Cited by
-
Impact of host physiology and external stressors on the bacterial community of Schmidtea mediterranea.Sci Rep. 2025 Feb 5;15(1):4398. doi: 10.1038/s41598-025-86920-0. Sci Rep. 2025. PMID: 39910204 Free PMC article.
References
-
- EU. (ed Council Regulation (EC)) (Official Journal of the European Union, L 142/1—L 142/739, 2008).
-
- Marone P. A., Hall W. C. & Hayes A. W. Reassessing the two-year rodent carcinogenicity bioassay: a review of the applicability to human risk and current perspectives. Regulatory toxicology and pharmacology: RTP 68, 108–118 (2014). - PubMed
-
- Heinonen T., Louekari K. & Tähti H. Need for Harmonized Strategies and Improved Assessment of Carcinogenic and Genotoxic Potencies of Chemical Substances. Journal of Translational Toxicology 1, 76–87 (2014).
-
- ICCVAM. ICCVAM Authorization Act of 2000. Public Law 106–545 (2000).
-
- Doktorova T. Y., Pauwels M., Vinken M., Vanhaecke T. & Rogiers V. Opportunities for an alternative integrating testing strategy for carcinogen hazard assessment? Critical Reviews in Toxicology 42, 91–106 (2012). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

