Reversal of Cocaine-Associated Synaptic Plasticity in Medial Prefrontal Cortex Parallels Elimination of Memory Retrieval
- PMID: 28466871
- PMCID: PMC5561348
- DOI: 10.1038/npp.2017.90
Reversal of Cocaine-Associated Synaptic Plasticity in Medial Prefrontal Cortex Parallels Elimination of Memory Retrieval
Abstract
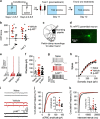
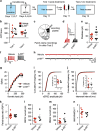
Addiction is characterized by abnormalities in prefrontal cortex that are thought to allow drug-associated cues to drive compulsive drug seeking and taking. Identification and reversal of these pathologic neuroadaptations are therefore critical for treatment of addiction. Previous studies using rodents reveal that drugs of abuse cause dendritic spine plasticity in prelimbic medial prefrontal cortex (PL-mPFC) pyramidal neurons, a phenomenon that correlates with the strength of drug-associated memories in vivo. Thus, we hypothesized that cocaine-evoked plasticity in PL-mPFC may underlie cocaine-associated memory retrieval, and therefore disruption of this plasticity would prevent retrieval. Indeed, using patch clamp electrophysiology we find that cocaine place conditioning increases excitatory presynaptic and postsynaptic transmission in rat PL-mPFC pyramidal neurons. This was accounted for by increases in excitatory presynaptic release, paired-pulse facilitation, and increased AMPA receptor transmission. Noradrenergic signaling is known to maintain glutamatergic plasticity upon reactivation of modified circuits, and we therefore next determined whether inhibition of noradrenergic signaling during memory reactivation would reverse the cocaine-evoked plasticity and/or disrupt the cocaine-associated memory. We find that administration of the β-adrenergic receptor antagonist propranolol before memory retrieval, but not after (during memory reconsolidation), reverses the cocaine-evoked presynaptic and postsynaptic modifications in PL-mPFC and causes long-lasting memory impairments. Taken together, these data reveal that cocaine-evoked synaptic plasticity in PL-mPFC is reversible in vivo, and suggest a novel strategy that would allow normalization of prefrontal circuitry in addiction.
Figures

Similar articles
-
Abstinence from Cocaine-Induced Conditioned Place Preference Produces Discrete Changes in Glutamatergic Synapses onto Deep Layer 5/6 Neurons from Prelimbic and Infralimbic Cortices.eNeuro. 2017 Dec 13;4(6):ENEURO.0308-17.2017. doi: 10.1523/ENEURO.0308-17.2017. eCollection 2017 Nov-Dec. eNeuro. 2017. PMID: 29242822 Free PMC article.
-
Neurobiological dissociation of retrieval and reconsolidation of cocaine-associated memory.J Neurosci. 2013 Jan 16;33(3):1271-81a. doi: 10.1523/JNEUROSCI.3463-12.2013. J Neurosci. 2013. PMID: 23325262 Free PMC article.
-
Cocaine exposure enhances excitatory synaptic drive to cholinergic neurons in the laterodorsal tegmental nucleus.Eur J Neurosci. 2013 Oct;38(7):3027-35. doi: 10.1111/ejn.12296. Epub 2013 Jul 3. Eur J Neurosci. 2013. PMID: 23822660
-
Cocaine-evoked synaptic plasticity of excitatory transmission in the ventral tegmental area.Cold Spring Harb Perspect Med. 2013 May 1;3(5):a012013. doi: 10.1101/cshperspect.a012013. Cold Spring Harb Perspect Med. 2013. PMID: 23637310 Free PMC article. Review.
-
Cortical mechanisms of cocaine sensitization.Crit Rev Neurobiol. 2005;17(2):69-86. doi: 10.1615/critrevneurobiol.v17.i2.20. Crit Rev Neurobiol. 2005. PMID: 16808728 Review.
Cited by
-
Memory retrieval in addiction: a role for miR-105-mediated regulation of D1 receptors in mPFC neurons projecting to the basolateral amygdala.BMC Biol. 2017 Dec 27;15(1):128. doi: 10.1186/s12915-017-0467-2. BMC Biol. 2017. PMID: 29282124 Free PMC article.
-
NMDA receptor-mediated synaptic transmission in prefrontal neurons underlies social memory retrieval in female mice.Neuropharmacology. 2022 Feb 15;204:108895. doi: 10.1016/j.neuropharm.2021.108895. Epub 2021 Nov 20. Neuropharmacology. 2022. PMID: 34813859 Free PMC article.
-
Effects of early life stress on cocaine conditioning and AMPA receptor composition are sex-specific and driven by TNF.Brain Behav Immun. 2019 May;78:41-51. doi: 10.1016/j.bbi.2019.01.006. Epub 2019 Jan 14. Brain Behav Immun. 2019. PMID: 30654007 Free PMC article.
-
Heterogeneity in the Paraventricular Thalamus: The Traffic Light of Motivated Behaviors.Front Behav Neurosci. 2020 Oct 16;14:590528. doi: 10.3389/fnbeh.2020.590528. eCollection 2020. Front Behav Neurosci. 2020. PMID: 33177999 Free PMC article.
-
The histone demethylase KDM6B in the medial prefrontal cortex epigenetically regulates cocaine reward memory.Neuropharmacology. 2018 Oct;141:113-125. doi: 10.1016/j.neuropharm.2018.08.030. Epub 2018 Aug 27. Neuropharmacology. 2018. PMID: 30165076 Free PMC article.
References
-
- Chen BT, Yau HJ, Hatch C, Kusumoto-Yoshida I, Cho SL, Hopf FW et al (2013). Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature 496: 359–362. - PubMed
-
- Childress AR, McLellan AT, O’Brien CP (1986). Role of conditioning factors in the development of drug dependence. Psychiatr Clin North Am 9: 413–425. - PubMed
-
- Dan Y, Poo MM (1992). Hebbian depression of isolated neuromuscular synapses in vitro. Science 256: 1570–1573. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

