Organocatalytic atroposelective synthesis of axially chiral styrenes
- PMID: 28466872
- PMCID: PMC5418600
- DOI: 10.1038/ncomms15238
Organocatalytic atroposelective synthesis of axially chiral styrenes
Erratum in
-
Erratum: Organocatalytic atroposelective synthesis of axially chiral styrenes.Nat Commun. 2017 Jun 27;8:16119. doi: 10.1038/ncomms16119. Nat Commun. 2017. PMID: 28653676 Free PMC article.
Abstract
Axially chiral compounds are widespread in biologically active compounds and are useful chiral ligands or organocatalysts in asymmetric catalysis. It is well-known that styrenes are one of the most abundant and principal feedstocks and thus represent excellent prospective building blocks for chemical synthesis. Driven by the development of atroposelective synthesis of axially chiral styrene derivatives, we discovered herein the asymmetric organocatalytic approach via direct Michael addition reaction of substituted diones/ketone esters/malononitrile to alkynals. The axially chiral styrene compounds were produced with good chemical yields, enantioselectivities and almost complete E/Z-selectivities through a secondary amine-catalysed iminium activation strategy under mild conditions. Such structural motifs are important precursors for further transformations into biologically active compounds and synthetic useful intermediates and may have potential applications in asymmetric synthesis as olefin ligands or organocatalysts.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
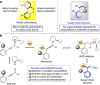



Similar articles
-
Construction of Axially Chiral Compounds via Asymmetric Organocatalysis.Acc Chem Res. 2018 Feb 20;51(2):534-547. doi: 10.1021/acs.accounts.7b00602. Epub 2018 Feb 8. Acc Chem Res. 2018. PMID: 29419282
-
Organocatalytic Enantioselective Construction of Axially Chiral Sulfone-Containing Styrenes.J Am Chem Soc. 2018 Jun 13;140(23):7056-7060. doi: 10.1021/jacs.8b03211. Epub 2018 May 31. J Am Chem Soc. 2018. PMID: 29781611
-
Organocatalytic Atroposelective Synthesis of Indole Derivatives Bearing Axial Chirality: Strategies and Applications.Acc Chem Res. 2022 Sep 20;55(18):2562-2580. doi: 10.1021/acs.accounts.2c00465. Epub 2022 Sep 2. Acc Chem Res. 2022. PMID: 36053083
-
Organocatalyzed Asymmetric Synthesis of Axially, Planar, and Helical Chiral Compounds.Chem Asian J. 2016 Feb 4;11(3):330-41. doi: 10.1002/asia.201500951. Epub 2015 Oct 15. Chem Asian J. 2016. PMID: 26395547 Review.
-
Recent advances in organocatalytic atroposelective reactions.Beilstein J Org Chem. 2025 Jan 9;21:55-121. doi: 10.3762/bjoc.21.6. eCollection 2025. Beilstein J Org Chem. 2025. PMID: 39811683 Free PMC article. Review.
Cited by
-
Diastereo- and atroposelective synthesis of N-arylpyrroles enabled by light-induced phosphoric acid catalysis.Nat Commun. 2023 Aug 9;14(1):4813. doi: 10.1038/s41467-023-40491-8. Nat Commun. 2023. PMID: 37558716 Free PMC article.
-
Organocatalytic enantioselective synthesis of Csp2-N atropisomers via formal Csp2-O bond amination.Chem Sci. 2024 Feb 9;15(11):3893-3900. doi: 10.1039/d3sc06707f. eCollection 2024 Mar 13. Chem Sci. 2024. PMID: 38487218 Free PMC article.
-
Discovery and synthesis of atropisomerically chiral acyl-substituted stable vinyl sulfoxonium ylides.Nat Chem. 2024 Jan;16(1):132-139. doi: 10.1038/s41557-023-01358-z. Epub 2023 Nov 9. Nat Chem. 2024. PMID: 37945832
-
Carbene-catalyzed atroposelective synthesis of axially chiral styrenes.Nat Commun. 2022 Jan 10;13(1):84. doi: 10.1038/s41467-021-27771-x. Nat Commun. 2022. PMID: 35013298 Free PMC article.
-
Enantioselective [3+3] atroposelective annulation catalyzed by N-heterocyclic carbenes.Nat Commun. 2018 Feb 9;9(1):611. doi: 10.1038/s41467-018-02952-3. Nat Commun. 2018. PMID: 29426851 Free PMC article.
References
-
- Bringmann G. et al.. Atroposelective synthesis of axially chiral biaryl compounds. Angew. Chem. Int. Ed. 44, 5384–5427 (2005). - PubMed
-
- Bringmann G., Gulder T., Gulder T. A. M. & Breuning M. Atroposelective total synthesis of axially chiral biaryl natural products. Chem. Rev. 111, 563–639 (2011). - PubMed
-
- Bencivenni G. Organocatalytic strategies for the synthesis of axially chiral compounds. Synlett 26, 1915–1922 (2015).
-
- Wencel-Delord J., Panossian A., Leroux F. R. & Colobert F. Recent advances and new concepts for the synthesis of axially stereoenriched biaryls. Chem. Soc. Rev. 44, 3418–3430 (2015). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

