Sarsasapogenin-AA13 ameliorates Aβ-induced cognitive deficits via improving neuroglial capacity on Aβ clearance and antiinflammation
- PMID: 28466999
- PMCID: PMC6492744
- DOI: 10.1111/cns.12697
Sarsasapogenin-AA13 ameliorates Aβ-induced cognitive deficits via improving neuroglial capacity on Aβ clearance and antiinflammation
Abstract
Aims: Sarsasapogenin has been reported to improve dementia symptoms somehow, probably through modulating the function of cholinergic system, suppressing neurofibrillary tangles, and inhibiting inflammation. However, the role of sarsasapogenin in response to beta-amyloid (Aβ) remains to be delineated. This study aimed to determine the therapeutic effect of sarsasapogenin-13 (AA13, a sarsasapogenin derivative) on learning and memory impairments in Aβ-injected mice, as well as the role of AA13 in neuroglia-mediated antiinflammation and Aβ clearance.
Methods: Focusing on the role of AA13 in regulating glial responses to Aβ, we conducted behavioral, morphological, and protein expression studies to explore the effects of AA13 on Aβ clearance and inflammatory regulation.
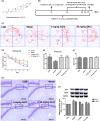
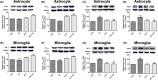
Results: The results indicated that oral administration of AA13 attenuated the memory deficits of intracerebroventricular (i.c.v.) Aβ-injected mice; also, AA13 protected neuroglial cells against Aβ-induced cytotoxicity. The further mechanical studies demonstrated that AA13 reversed the upregulation of proinflammatory M1 markers and increased the expression of antiinflammatory M2 markers in Aβ-treated cells. Furthermore, AA13 facilitated Aβ clearance through promoting Aβ phagocytosis and degradation. AA13 modulated the expression of fatty acid translocase (CD36), insulin-degrading enzyme (IDE), neprilysin (NEP), and endothelin-converting enzyme (ECE) in neuroglia.
Conclusion: The present study indicated that the neuroprotective effect of AA13 might relate to its modulatory effects on microglia activation state, phagocytic ability, and expression of Aβ-degrading enzymes, which makes it a promising therapeutic agent in the early stage of Alzheimer's disease (AD).
Keywords: antiinflammation; beta-amyloid clearance; neuroglia; phagocytosis; sarsasapogenin-AA13.
© 2017 John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no competing interests.
Figures




Similar articles
-
A multifunctional peptide rescues memory deficits in Alzheimer's disease transgenic mice by inhibiting Aβ42-induced cytotoxicity and increasing microglial phagocytosis.Neurobiol Dis. 2012 Jun;46(3):701-9. doi: 10.1016/j.nbd.2012.03.013. Epub 2012 Mar 9. Neurobiol Dis. 2012. PMID: 22426388
-
5-N-ethyl Carboxamidoadenosine Stimulates Adenosine-2b Receptor-Mediated Mitogen-Activated Protein Kinase Pathway to Improve Brain Mitochondrial Function in Amyloid Beta-Induced Cognitive Deficit Mice.Neuromolecular Med. 2020 Dec;22(4):542-556. doi: 10.1007/s12017-020-08615-1. Epub 2020 Sep 14. Neuromolecular Med. 2020. PMID: 32926328
-
A validated UPLC-MS/MS method for quantitative determination of a potent neuroprotective agent Sarsasapogenin-AA13 in rat plasma: Application to pharmacokinetic studies.Biomed Chromatogr. 2020 Mar;34(3):e4775. doi: 10.1002/bmc.4775. Epub 2020 Jan 17. Biomed Chromatogr. 2020. PMID: 31845362
-
Aβ degradation or cerebral perfusion? Divergent effects of multifunctional enzymes.Front Aging Neurosci. 2014 Sep 11;6:238. doi: 10.3389/fnagi.2014.00238. eCollection 2014. Front Aging Neurosci. 2014. PMID: 25309424 Free PMC article. Review.
-
Chemistry, Biosynthesis and Pharmacology of Sarsasapogenin: A Potential Natural Steroid Molecule for New Drug Design, Development and Therapy.Molecules. 2022 Mar 21;27(6):2032. doi: 10.3390/molecules27062032. Molecules. 2022. PMID: 35335393 Free PMC article. Review.
Cited by
-
Glibenclamide-Loaded Engineered Nanovectors (GNVs) Modulate Autophagy and NLRP3-Inflammasome Activation.Pharmaceuticals (Basel). 2023 Dec 13;16(12):1725. doi: 10.3390/ph16121725. Pharmaceuticals (Basel). 2023. PMID: 38139851 Free PMC article.
-
Sarsasapogenin Suppresses RANKL-Induced Osteoclastogenesis in vitro and Prevents Lipopolysaccharide-Induced Bone Loss in vivo.Drug Des Devel Ther. 2020 Aug 24;14:3435-3447. doi: 10.2147/DDDT.S256867. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 32943842 Free PMC article.
-
Chronic cerebral hypoperfusion and blood-brain barrier disruption in uninjured brain areas of rhesus monkeys subjected to transient ischemic stroke.J Cereb Blood Flow Metab. 2022 Jul;42(7):1335-1346. doi: 10.1177/0271678X221078065. Epub 2022 Feb 9. J Cereb Blood Flow Metab. 2022. PMID: 35137610 Free PMC article.
-
Pathway-based network medicine identifies novel natural products for Alzheimer's disease.Alzheimers Res Ther. 2025 Feb 14;17(1):43. doi: 10.1186/s13195-025-01694-x. Alzheimers Res Ther. 2025. PMID: 39953559 Free PMC article.
-
Icariin Attenuates M1 Activation of Microglia and Aβ Plaque Accumulation in the Hippocampus and Prefrontal Cortex by Up-Regulating PPARγ in Restraint/Isolation-Stressed APP/PS1 Mice.Front Neurosci. 2019 Mar 28;13:291. doi: 10.3389/fnins.2019.00291. eCollection 2019. Front Neurosci. 2019. PMID: 31001073 Free PMC article.
References
-
- Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741‐766. - PubMed
-
- Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010;362:329‐344. - PubMed
-
- Biswas SK, Chittezhath M, Shalova IN, Lim JY. Macrophage polarization and plasticity in health and disease. Immunol Res. 2012;53:11‐24. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

