Palladium-Catalyzed Enantioselective Redox-Relay Heck Alkynylation of Alkenols To Access Propargylic Stereocenters
- PMID: 28467031
- PMCID: PMC5528850
- DOI: 10.1002/anie.201703089
Palladium-Catalyzed Enantioselective Redox-Relay Heck Alkynylation of Alkenols To Access Propargylic Stereocenters
Abstract
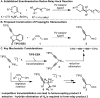
An enantioselective redox-relay Heck alkynylation of di- and trisubstituted alkenols to construct propargylic stereocenters is disclosed using a new pyridine oxazoline ligand. This strategy allows direct access to chiral β-alkynyl carbonyl compounds employing allylic alcohol substrates in contrast to more traditional conjugate addition methods.
Keywords: Heck reaction; alkenes; alkynylation; propargylic stereocenter.
© 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
-
For selected reviews, see:
- Heck RF. Acc Chem Res. 1979;12:146–151.
- Crisp GT. Chem Soc Rev. 1998;27:427–436.
- Beletskaya IP, Cheprakov AV. Chem Rev. 2000;100:3009–3066. - PubMed
- Shibasaki M, Vogl EM, Ohshima T. Adv Synth Catal. 2004;346:1533–1552.
- Zeni G, Larock RC. Chem Rev. 2006;106:4644–4680. - PubMed
- Le Bras J, Muzart J. Chem Rev. 2011;111:1170–1214. - PubMed
- McCartney D, Guiry PJ. Chem Soc Rev. 2011;40:5122–5150. - PubMed
- Seechurn CCCJ, Kitching MO, Colacot TJ, Snieckus V. Angew Chem. 2012;124:5150–5174.
- Angew Chem Int Ed. 2012;51:5062–5085. - PubMed
-
-
-
For selected works, see:
- Larock RC, Leung WY, Stolz-Dunn S. Tetrahedron Lett. 1989;30:6629–6632.
- Bouquillon S, Ganchegui B, Estrine B, Hénin F, Muzart J. J Organomet Chem. 2001;634:153–156.
- Berthiol F, Doucet H, Santelli M. Tetrahedron. 2006;62:4372–4383.
- Oliveira CC, Pfaltz A, Correia CRD. Angew Chem. 2015;127:14242–14245.
- Angew Chem Int Ed. 2015;54:14036–14039. - PubMed
- Singh S, Bruffaerts J, Vasseur A, Marek I. Nat Commun. 2017;8 doi: 10.1038/ncomms14200. - DOI - PMC - PubMed
-
-
- Mei TS, Werner EW, Burckle AJ, Sigman MS. J Am Chem Soc. 2013;135:6830–6833. - PMC - PubMed
- Mei TS, Patel HH, Sigman MS. Nature. 2014;508:340–344. - PMC - PubMed
- Zhang C, Santiago CB, Kou L, Sigman MS. J Am Chem Soc. 2015;137:7290–7293. - PMC - PubMed
- Chen ZM, Hilton MJ, Sigman MS. J Am Chem Soc. 2016;138:11461–11464. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources