Pre-clinical and clinical investigations of metabolic zonation in liver diseases: The potential of microphysiology systems
- PMID: 28467181
- PMCID: PMC5661767
- DOI: 10.1177/1535370217707731
Pre-clinical and clinical investigations of metabolic zonation in liver diseases: The potential of microphysiology systems
Abstract
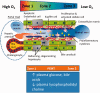
The establishment of metabolic zonation within a hepatic lobule ascribes specific functions to hepatocytes based on unique, location-dependent gene expression patterns. Recently, there have been significant developments in the field of metabolic liver zonation. A little over a decade ago, the role of β-catenin signaling was identified as a key regulator of gene expression and function in pericentral hepatocytes. Since then, additional molecules have been identified that regulate the pattern of Wnt/β-catenin signaling within a lobule and determine gene expression and function in other hepatic zones. Currently, the molecular basis of metabolic zonation in the liver appears to be a 'push and pull' between signaling pathways. Such compartmentalization not only provides an efficient assembly line for hepatocyte functions but also can account for restricting the initial hepatic damage and pathology from some hepatotoxic drugs to specific zones, possibly enabling effective regeneration and restitution responses from unaffected cells. Careful analysis and experimentation have also revealed that many pathological conditions in the liver lobule are spatially heterogeneous. We will review current research efforts that have focused on examination of the role and regulation of such mechanisms of hepatocyte adaptation and repair. We will discuss how the pathological organ-specific microenvironment affects cell signaling and metabolic liver zonation, especially in steatosis, viral hepatitis, and hepatocellular carcinoma. We will discuss how the use of new human microphysiological platforms will lead to a better understanding of liver disease progression, diagnosis, and therapies. In conclusion, we aim to provide insights into the role and regulation of metabolic zonation and function using traditional and innovative approaches. Impact statement Liver zonation of oxygen tension along the liver sinusoids has been identified as a critical liver microenvironment that impacts specific liver functions such as intermediary metabolism of amino acids, lipids, and carbohydrates, detoxification of xenobiotics and as sites for initiation of liver diseases. To date, most information on the role of zonation in liver disease including, non-alcoholic fatty liver disease (NAFLD), non-alcoholic steatohepatitis (NASH), cirrhosis, and hepatocellular carcinoma (HCC) have been obtained from animal models. It is now possible to complement animal studies with human liver, microphysiology systems (MPS) containing induced pluripotent stem cells engineered to create disease models where it is also possible to control the in vitro liver oxygen microenvironment to define the role of zonation on the mechanism(s) of disease progression. The field now has the tools to investigate human liver disease progression, diagnosis, and therapeutic development.
Keywords: Liver zonation; hepatocytes; induced pluripotent stem cells; liver diseases; microfluidics; microphysiology systems.
Figures



Similar articles
-
Transcription dynamics in a physiological process: β-catenin signaling directs liver metabolic zonation.Int J Biochem Cell Biol. 2011 Feb;43(2):271-8. doi: 10.1016/j.biocel.2009.11.004. Epub 2009 Nov 13. Int J Biochem Cell Biol. 2011. PMID: 19914393 Review.
-
Control of oxygen tension recapitulates zone-specific functions in human liver microphysiology systems.Exp Biol Med (Maywood). 2017 Oct;242(16):1617-1632. doi: 10.1177/1535370217703978. Epub 2017 Apr 14. Exp Biol Med (Maywood). 2017. PMID: 28409533 Free PMC article.
-
Dual modulation of human hepatic zonation via canonical and non-canonical Wnt pathways.Exp Mol Med. 2017 Dec 15;49(12):e413. doi: 10.1038/emm.2017.226. Exp Mol Med. 2017. PMID: 29244788 Free PMC article.
-
Hepatitis C viral proteins perturb metabolic liver zonation.J Hepatol. 2015 Feb;62(2):278-85. doi: 10.1016/j.jhep.2014.09.004. Epub 2014 Sep 16. J Hepatol. 2015. PMID: 25220251
-
Wnt/beta-catenin signaling and its modulators in nonalcoholic fatty liver diseases.Hepatobiliary Pancreat Dis Int. 2023 Aug;22(4):333-345. doi: 10.1016/j.hbpd.2022.10.003. Epub 2022 Oct 20. Hepatobiliary Pancreat Dis Int. 2023. PMID: 36448560 Review.
Cited by
-
Human biomimetic liver microphysiology systems in drug development and precision medicine.Nat Rev Gastroenterol Hepatol. 2021 Apr;18(4):252-268. doi: 10.1038/s41575-020-00386-1. Epub 2020 Dec 17. Nat Rev Gastroenterol Hepatol. 2021. PMID: 33335282 Free PMC article. Review.
-
The Ploidy State as a Determinant of Hepatocyte Proliferation.Semin Liver Dis. 2023 Nov;43(4):460-471. doi: 10.1055/a-2211-2144. Epub 2023 Nov 15. Semin Liver Dis. 2023. PMID: 37967885 Free PMC article. Review.
-
Analysis of the nitric oxide-cyclic guanosine monophosphate pathway in experimental liver cirrhosis suggests phosphodiesterase-5 as potential target to treat portal hypertension.World J Gastroenterol. 2018 Oct 14;24(38):4356-4368. doi: 10.3748/wjg.v24.i38.4356. World J Gastroenterol. 2018. PMID: 30344420 Free PMC article.
-
Modeling the Effect of the Metastatic Microenvironment on Phenotypes Conferred by Estrogen Receptor Mutations Using a Human Liver Microphysiological System.Sci Rep. 2019 Jun 6;9(1):8341. doi: 10.1038/s41598-019-44756-5. Sci Rep. 2019. PMID: 31171849 Free PMC article.
-
Harnessing Human Microphysiology Systems as Key Experimental Models for Quantitative Systems Pharmacology.Handb Exp Pharmacol. 2019;260:327-367. doi: 10.1007/164_2019_239. Handb Exp Pharmacol. 2019. PMID: 31201557 Free PMC article.
References
-
- Grompe M. The origin of hepatocytes. Gastroenterology 2005; 128: 2158–60. - PubMed
-
- Bahar Halpern K, Shenhav R, Matcovitch-Natan O, Toth B, Lemze D, Golan M, Massasa EE, Baydatch S, Landen S, Moor AE, Brandis A, Giladi A, Stokar-Avihail A, David E, Amit I, Itzkovitz S. Single-cell spatial reconstruction reveals global division of labour in the mammalian liver. Nature 2017; 542: 352–6. - PMC - PubMed
-
- Birchmeier W. Orchestrating Wnt signalling for metabolic liver zonation. Nat Cell Biol 2016; 18: 463–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

