16S rRNA gene sequencing and healthy reference ranges for 28 clinically relevant microbial taxa from the human gut microbiome
- PMID: 28467461
- PMCID: PMC5414997
- DOI: 10.1371/journal.pone.0176555
16S rRNA gene sequencing and healthy reference ranges for 28 clinically relevant microbial taxa from the human gut microbiome
Erratum in
-
Correction: 16S rRNA gene sequencing and healthy reference ranges for 28 clinically relevant microbial taxa from the human gut microbiome.PLoS One. 2019 Feb 12;14(2):e0212474. doi: 10.1371/journal.pone.0212474. eCollection 2019. PLoS One. 2019. PMID: 30753236 Free PMC article.
Expression of concern in
-
Expression of Concern: 16S rRNA gene sequencing and healthy reference ranges for 28 clinically relevant microbial taxa from the human gut microbiome.PLoS One. 2022 Oct 20;17(10):e0276752. doi: 10.1371/journal.pone.0276752. eCollection 2022. PLoS One. 2022. PMID: 36264907 Free PMC article. No abstract available.
Abstract
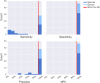
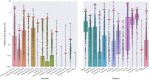
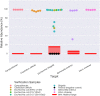
Changes in the relative abundances of many intestinal microorganisms, both those that naturally occur in the human gut microbiome and those that are considered pathogens, have been associated with a range of diseases. To more accurately diagnose health conditions, medical practitioners could benefit from a molecular, culture-independent assay for the quantification of these microorganisms in the context of a healthy reference range. Here we present the targeted sequencing of the microbial 16S rRNA gene of clinically relevant gut microorganisms as a method to provide a gut screening test that could assist in the clinical diagnosis of certain health conditions. We evaluated the possibility of detecting 46 clinical prokaryotic targets in the human gut, 28 of which could be identified with high precision and sensitivity by a bioinformatics pipeline that includes sequence analysis and taxonomic annotation. These targets included 20 commensal, 3 beneficial (probiotic), and 5 pathogenic intestinal microbial taxa. Using stool microbiome samples from a cohort of 897 healthy individuals, we established a reference range defining clinically relevant relative levels for each of the 28 targets. Our assay quantifies 28 targets in the context of a healthy reference range and correctly reflected 38/38 verification samples of real and synthetic stool material containing known gut pathogens. Thus, we have established a method to determine microbiome composition with a focus on clinically relevant taxa, which has the potential to contribute to patient diagnosis, treatment, and monitoring. More broadly, our method can facilitate epidemiological studies of the microbiome as it relates to overall human health and disease.
Conflict of interest statement
Figures


References
-
- LeBlanc JG, Milani C, de Giori GS, Sesma F, van Sinderen D, Ventura M. Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr Opin in Biotechnol. 2013;24: 160–168. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

