Outcomes and prognoses of patients with ovarian cancer using bevacizumab: 6-year experience in a tertiary care hospital of northern Taiwan
- PMID: 28467466
- PMCID: PMC5415172
- DOI: 10.1371/journal.pone.0175703
Outcomes and prognoses of patients with ovarian cancer using bevacizumab: 6-year experience in a tertiary care hospital of northern Taiwan
Abstract
Purpose: Bevacizumab (BEV) has been used for ovarian cancer (OC) for years in Taiwan, but the associated data related to outcome is scant. This retrospective study reviewed patients with OC treated with BEV and analyzed their results.
Patients and methods: All patients with OC treated with BEV from 2009 to 2015 in the Linkou branch of Chang Gung Memorial Hospital in Northern Taiwan were included. According to the means of administration, the patients were classified into 6 groups as follows: A-BEV plus chemotherapy (C/T) for initial platinum-resistant (PR) recurrent OC, B-BEV plus C/T for initial platinum-sensitive (PS) recurrent OC, C-BEV alone for recurrent OC, D-BEV plus 1st adjuvant C/T, E-BEV plus neoadjuvant C/T, and F-intraperitoneal (IP) BEV. Progression-free survival (PFS), overall survival (OS), hazard ratios (HRs), overall response rate (ORR), and mean number of BEV cycles were analyzed for groups A to E. Clinical improvement of ascites was assessed for group F.
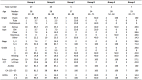
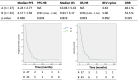
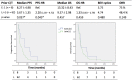
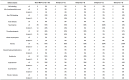
Results: A comparison of early use (only one round of prior C/T) versus late use (multiple rounds of prior C/T) in patients of groups A and B showed a superior PFS (8.27 vs. 3.67, p = 0.037) in the early use group. No significant differences were found between groups A and B (PFS: 4.24 vs. 4.17 months, p = 0.690; OS: 10.06 vs. 9.93 months, p = 0.819; mean BEV cycles: 4.63 vs. 5.0 p = 0.992; ORR: 48.1% vs. 53.5%, p = 0.425). Comparing the response and non-response subgroups of patients in groups A and B, a better outcome was associated with endometrioid type cell (HR = 0.28, p = 0.008), good ECOG performance status (HR = 0.51, p = 0.005), and lack of ascites (HR = 0.67, p = 0.004). Comparing group C with groups A plus B, the BEV alone group had a poorer PFS (1.02 VS. 4.19, p = 0.04) and OS (1.42 VS. 9.99 p = 0.001) than the BEV plus C/T group. In group F, a good clinical benefit rate (85.6%) of ascites improvement was noted. Two patients had grade 5 gastrointestinal bleeding and venous/arterial thromboembolic events after administration of BEV. Grade 3 neutropenia and thrombocytopenia occurred more frequently in our study.
Conclusion: Early use of BEV combined with chemotherapy had a significant benefit in PFS for patients with recurrent OC. BEV plus chemotherapy was better than BEV alone for recurrent OC. In addition, IP BEV was helpful for improving clinical ascites.
Conflict of interest statement
Figures

References
-
- American Cancer Society. Ovarian cancer. 358 http://www.cancer.org/cancer/359ovariancancer/detailedguide/ovarian-canc.... Accessed March 24, 360 2016
-
- Taiwan DoH, Executive Yuan. Cancer Registry Annual Report, 2009. 2011.
-
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108 - DOI - PubMed
-
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76. doi: 10.1038/nm0603-669 - DOI - PubMed
-
- Ferrara N. VEGF as a therapeutic target in cancer. Oncology. 2005;69 Suppl 3:11–6. doi: 10.1159/000088479 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

