Spinal Transection Alters External Urethral Sphincter Activity during Spontaneous Voiding in Freely Moving Rats
- PMID: 28467736
- PMCID: PMC5661870
- DOI: 10.1089/neu.2016.4844
Spinal Transection Alters External Urethral Sphincter Activity during Spontaneous Voiding in Freely Moving Rats
Abstract
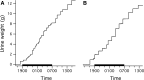
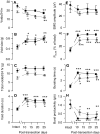
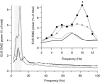
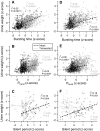
The rat is a commonly used model for the study of lower urinary tract function before and after spinal cord injury. We have previously reported that in unanesthetized freely moving rats, although phasic external urethral sphincter (EUS) activity (bursting) is most common during micturition, productive voiding can occur in the absence of bursting, which differs from results seen in anesthetized or unanesthetized restrained animals. The purpose of the present study was to characterize EUS behavior in unanesthetized, freely moving rats before and after mid-thoracic (T8) or thoraco-lumbar (T13-L1) spinal transection to determine how EUS behavior after spinal cord injury differs from that seen in anesthetized or unanesthetized restrained rats. Several abnormalities became evident that were comparable after transection at either level, including the following: repetitive non-voiding EUS contractions; increased prevalence, intensity, and duration of EUS bursting; decreased rate of urine evacuation during bursting; increased void size and decreased number of daily voids; shorter inter-burst silent period and increased frequency of bursting; and loss of the direct linear relationships that are evident in intact animals between void size and bursting silent period. These data suggest that transection-induced delayed initiation of EUS bursting allows co-contraction of the bladder and the EUS that prevents or limits urine evacuation, resulting in a detrusor-sphincter dyssynergia-like phenomenon. In addition, the higher-than-normal frequency at which EUS bursting occurs after transection is associated with shorter silent periods during which urine typically flows, which interferes with voiding by slowing the rate of urine evacuation. That results were comparable after either transection suggests that the central pattern generator responsible for EUS bursting is located caudal to the L1 spinal segment.
Keywords: EMG; external urethral sphincter; implanted electrodes; longitudinal study; spinal cord.
Conflict of interest statement
No competing financial interests exist.
Figures




Similar articles
-
Contribution of the external urethral sphincter to urinary void size in unanesthetized unrestrained rats.Neurourol Urodyn. 2016 Aug;35(6):696-702. doi: 10.1002/nau.22789. Epub 2015 May 20. Neurourol Urodyn. 2016. PMID: 25995074 Free PMC article.
-
Characterization of bladder and external urethral activity in mice with or without spinal cord injury--a comparison study with rats.Am J Physiol Regul Integr Comp Physiol. 2016 Apr 15;310(8):R752-8. doi: 10.1152/ajpregu.00450.2015. Epub 2016 Jan 27. Am J Physiol Regul Integr Comp Physiol. 2016. PMID: 26818058 Free PMC article.
-
Quantification of external urethral sphincter and bladder activity during micturition in the intact and spinally transected adult rat.Exp Neurol. 2011 Mar;228(1):59-68. doi: 10.1016/j.expneurol.2010.12.008. Epub 2010 Dec 15. Exp Neurol. 2011. PMID: 21167152
-
Effect of injury severity on lower urinary tract function after experimental spinal cord injury.Prog Brain Res. 2006;152:117-34. doi: 10.1016/S0079-6123(05)52008-9. Prog Brain Res. 2006. PMID: 16198697 Review.
-
Spinal mechanisms contributing to urethral striated sphincter control during continence and micturition: "how good things might go bad".Prog Brain Res. 2006;152:85-95. doi: 10.1016/S0079-6123(05)52006-5. Prog Brain Res. 2006. PMID: 16198695 Review.
Cited by
-
Upregulated 5-HT1A Receptors Regulate Lower Urinary Tract Function in Rats after Complete Spinal Cord Injury.J Neurotrauma. 2023 May;40(9-10):845-861. doi: 10.1089/neu.2022.0329. Epub 2023 Mar 14. J Neurotrauma. 2023. PMID: 36762948 Free PMC article.
-
AAV Vector Mediated Delivery of NG2 Function Neutralizing Antibody and Neurotrophin NT-3 Improves Synaptic Transmission, Locomotion, and Urinary Tract Function after Spinal Cord Contusion Injury in Adult Rats.J Neurosci. 2023 Mar 1;43(9):1492-1508. doi: 10.1523/JNEUROSCI.1276-22.2023. Epub 2023 Jan 18. J Neurosci. 2023. PMID: 36653191 Free PMC article.
-
Improvement of lower urinary tract function by a selective serotonin 5-HT1A receptor agonist, NLX-112, after chronic spinal cord injury.Exp Neurol. 2020 Oct;332:113395. doi: 10.1016/j.expneurol.2020.113395. Epub 2020 Jun 30. Exp Neurol. 2020. PMID: 32615138 Free PMC article.
-
Enkephalinergic Neurons in Barrington's Nucleus Gate Sex-Specific Control of Micturition.Res Sq [Preprint]. 2025 Jul 2:rs.3.rs-6940959. doi: 10.21203/rs.3.rs-6940959/v1. Res Sq. 2025. PMID: 40630540 Free PMC article. Preprint.
-
Modulatory effects of intravesical P2X2/3 purinergic receptor inhibition on lower urinary tract electromyographic properties and voiding function of female rats with moderate or severe spinal cord injury.BJU Int. 2019 Mar;123(3):538-547. doi: 10.1111/bju.14561. Epub 2018 Oct 26. BJU Int. 2019. PMID: 30255543 Free PMC article.
References
-
- Kruse M.N., Belton A.L., and de Groat W.C. (1993). Changes in bladder and external urethral sphincter function after spinal cord injury in the rat. Am. J. Physiol. 264, R1157–1163 - PubMed
-
- Weld K.J., Graney M.J., and Dmochowski R.R. (2000). Clinical significance of detrusor sphincter dyssynergia type in patients with post-traumatic spinal cord injury. Urology 56, 565–568 - PubMed
-
- Swartz D. (1946). The neurogenic bladder in spinal cord injury. Can. Med. Assoc. J. 54, 333–339 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

