Sphingosine kinase 2 inhibition synergises with bortezomib to target myeloma by enhancing endoplasmic reticulum stress
- PMID: 28467788
- PMCID: PMC5546428
- DOI: 10.18632/oncotarget.17115
Sphingosine kinase 2 inhibition synergises with bortezomib to target myeloma by enhancing endoplasmic reticulum stress
Abstract
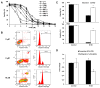
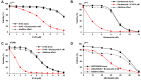
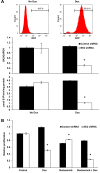
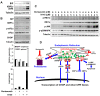
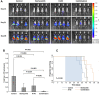
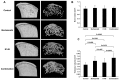
The proteasome inhibitor bortezomib has proven to be invaluable in the treatment of myeloma. By exploiting the inherent high immunoglobulin protein production of malignant plasma cells, bortezomib induces endoplasmic reticulum (ER) stress and the unfolded protein response (UPR), resulting in myeloma cell death. In most cases, however, the disease remains incurable highlighting the need for new therapeutic targets. Sphingosine kinase 2 (SK2) has been proposed as one such therapeutic target for myeloma. Our observations that bortezomib and SK2 inhibitors independently elicited induction of ER stress and the UPR prompted us to examine potential synergy between these agents in myeloma. Targeting SK2 synergistically contributed to ER stress and UPR activation induced by bortezomib, as evidenced by activation of the IRE1 pathway and stress kinases JNK and p38MAPK, thereby resulting in potent synergistic myeloma apoptosis in vitro. The combination of bortezomib and SK2 inhibition also exhibited strong in vivo synergy and favourable effects on bone disease. Therefore, our studies suggest that perturbations of sphingolipid signalling can synergistically enhance the effects seen with proteasome inhibition, highlighting the potential for the combination of these two modes of increasing ER stress to be formally evaluated in clinical trials for the treatment of myeloma patients.
Keywords: endoplasmic reticulum; myeloma; proteasome inhibitor; sphingosine kinase.
Conflict of interest statement
The authors have no conflicts-of-interest to disclose.
Figures


Similar articles
-
Resensitising proteasome inhibitor-resistant myeloma with sphingosine kinase 2 inhibition.Neoplasia. 2022 Jan;24(1):1-11. doi: 10.1016/j.neo.2021.11.009. Epub 2021 Nov 23. Neoplasia. 2022. PMID: 34826777 Free PMC article.
-
The novel β2-selective proteasome inhibitor LU-102 synergizes with bortezomib and carfilzomib to overcome proteasome inhibitor resistance of myeloma cells.Haematologica. 2015 Oct;100(10):1350-60. doi: 10.3324/haematol.2014.109421. Epub 2015 Jun 11. Haematologica. 2015. PMID: 26069288 Free PMC article.
-
Therapeutic enhancement of ER stress by insulin-like growth factor I sensitizes myeloma cells to proteasomal inhibitors.Clin Cancer Res. 2013 Jul 1;19(13):3556-66. doi: 10.1158/1078-0432.CCR-12-3134. Epub 2013 May 14. Clin Cancer Res. 2013. PMID: 23674497
-
Endoplasmic-reticulum stress pathway-associated mechanisms of action of proteasome inhibitors in multiple myeloma.Int J Hematol. 2016 Sep;104(3):273-80. doi: 10.1007/s12185-016-2016-0. Epub 2016 May 12. Int J Hematol. 2016. PMID: 27169614 Review.
-
Endoplasmic reticulum stress and proteasome inhibitors in multiple myeloma: room for improvement.Pol Arch Intern Med. 2021 Apr 29;131(4):361-368. doi: 10.20452/pamw.15896. Epub 2021 Mar 26. Pol Arch Intern Med. 2021. PMID: 33768999 Review.
Cited by
-
The fatty acid elongase ELOVL6 regulates bortezomib resistance in multiple myeloma.Blood Adv. 2021 Apr 13;5(7):1933-1946. doi: 10.1182/bloodadvances.2020002578. Blood Adv. 2021. PMID: 33821992 Free PMC article.
-
Formononetin Regulates Multiple Oncogenic Signaling Cascades and Enhances Sensitivity to Bortezomib in a Multiple Myeloma Mouse Model.Biomolecules. 2019 Jul 7;9(7):262. doi: 10.3390/biom9070262. Biomolecules. 2019. PMID: 31284669 Free PMC article.
-
Enhancing ER stress in myeloma.Aging (Albany NY). 2017 Jul 30;9(7):1645-1646. doi: 10.18632/aging.101273. Aging (Albany NY). 2017. PMID: 28758893 Free PMC article. No abstract available.
-
The combination of the tubulin binding small molecule PTC596 and proteasome inhibitors suppresses the growth of myeloma cells.Sci Rep. 2021 Jan 22;11(1):2074. doi: 10.1038/s41598-021-81577-x. Sci Rep. 2021. PMID: 33483574 Free PMC article.
-
Advancements on the Multifaceted Roles of Sphingolipids in Hematological Malignancies.Int J Mol Sci. 2022 Oct 22;23(21):12745. doi: 10.3390/ijms232112745. Int J Mol Sci. 2022. PMID: 36361536 Free PMC article. Review.
References
-
- Rollig C, Knop S, Bornhauser M. Multiple myeloma. Lancet. 2015;385:2197–208. - PubMed
-
- Kyle RA, Rajkumar SV. An overview of the progress in the treatment of multiple myeloma. Expert Rev Hematol. 2014;7:5–7. - PubMed
-
- Vincenz L, Jager R, O’Dwyer M, Samali A. Endoplasmic reticulum stress and the unfolded protein response: targeting the Achilles heel of multiple myeloma. Mol Cancer Ther. 2013;12:831–43. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

