The antigen-binding fragment of human gamma immunoglobulin prevents amyloid β-peptide folding into β-sheet to form oligomers
- PMID: 28467807
- PMCID: PMC5522293
- DOI: 10.18632/oncotarget.17074
The antigen-binding fragment of human gamma immunoglobulin prevents amyloid β-peptide folding into β-sheet to form oligomers
Abstract
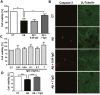
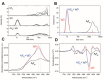
The amyloid beta-peptide (Aβ) plays a leading role in Alzheimer's disease (AD) physiopathology. Even though monomeric forms of Aβ are harmless to cells, Aβ can aggregate into β-sheet oligomers and fibrils, which are both neurotoxic. Therefore, one of the main therapeutic approaches to cure or delay AD onset and progression is targeting Aβ aggregation. In the present study, we show that a pool of human gamma immunoglobulins (IgG) protected cortical neurons from the challenge with Aβ oligomers, as assayed by MTT reduction, caspase-3 activation and cytoskeleton integrity. In addition, we report the inhibitory effect of IgG on Aβ aggregation, as shown by Thioflavin T assay, size exclusion chromatography and atomic force microscopy. Similar results were obtained with Palivizumab, a human anti-sincitial virus antibody. In order to dissect the important domains, we cleaved the pool of human IgG with papain to obtain Fab and Fc fragments. Using these cleaved fragments, we functionally identified Fab as the immunoglobulin fragment inhibiting Aβ aggregation, a result that was further confirmed by an in silico structural model. Interestingly, bioinformatic tools show a highly conserved structure able to bind amyloid in the Fab region. Overall, our data strongly support the inhibitory effect of human IgG on Aβ aggregation and its neuroprotective role.
Keywords: Alzheimer’s disease; Fab; amyloid; immunoglobulin; oligomers.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures



Similar articles
-
Unmodified and pyroglutamylated amyloid β peptides form hypertoxic hetero-oligomers of unique secondary structure.FEBS J. 2017 May;284(9):1355-1369. doi: 10.1111/febs.14058. Epub 2017 Apr 10. FEBS J. 2017. PMID: 28294556
-
Thioflavin T templates amyloid β(1-40) conformation and aggregation pathway.Biophys Chem. 2015 Nov;206:1-11. doi: 10.1016/j.bpc.2015.06.006. Epub 2015 Jun 16. Biophys Chem. 2015. PMID: 26100600
-
α-Sheet secondary structure in amyloid β-peptide drives aggregation and toxicity in Alzheimer's disease.Proc Natl Acad Sci U S A. 2019 Apr 30;116(18):8895-8900. doi: 10.1073/pnas.1820585116. Epub 2019 Apr 19. Proc Natl Acad Sci U S A. 2019. PMID: 31004062 Free PMC article.
-
[Amyloid-selective Photooxygenation toward Treatment for Amyloid Diseases].Yakugaku Zasshi. 2018;138(1):47-53. doi: 10.1248/yakushi.17-00186-2. Yakugaku Zasshi. 2018. PMID: 29311465 Review. Japanese.
-
Inhibition of amyloid-β aggregation in Alzheimer's disease.Curr Pharm Des. 2014;20(8):1223-43. doi: 10.2174/13816128113199990068. Curr Pharm Des. 2014. PMID: 23713775 Review.
Cited by
-
The Functional Roles and Applications of Immunoglobulins in Neurodegenerative Disease.Int J Mol Sci. 2020 Jul 26;21(15):5295. doi: 10.3390/ijms21155295. Int J Mol Sci. 2020. PMID: 32722559 Free PMC article. Review.
-
Differential regulation of insulin signalling by monomeric and oligomeric amyloid beta-peptide.Brain Commun. 2022 Sep 24;4(5):fcac243. doi: 10.1093/braincomms/fcac243. eCollection 2022. Brain Commun. 2022. PMID: 36267327 Free PMC article.
-
Decoding the molecular and structural determinants of the neurokinin A and Aβ1-42 peptide cross-interaction in the amyloid cascade pathway.iScience. 2024 Oct 16;27(11):111187. doi: 10.1016/j.isci.2024.111187. eCollection 2024 Nov 15. iScience. 2024. PMID: 39559760 Free PMC article.
-
The Dual Role of Amyloid Beta-Peptide in Oxidative Stress and Inflammation: Unveiling Their Connections in Alzheimer's Disease Etiopathology.Antioxidants (Basel). 2024 Oct 8;13(10):1208. doi: 10.3390/antiox13101208. Antioxidants (Basel). 2024. PMID: 39456461 Free PMC article. Review.
-
Mitostasis, Calcium and Free Radicals in Health, Aging and Neurodegeneration.Biomolecules. 2021 Jul 10;11(7):1012. doi: 10.3390/biom11071012. Biomolecules. 2021. PMID: 34356637 Free PMC article. Review.
References
-
- Talantova M, Sanz-Blasco S, Zhang X, Xia P, Akhtar MW, Okamoto S, Dziewczapolski G, Nakamura T, Cao G, Pratt AE, Kang YJ, Tu S, Molokanova E, et al. A induces astrocytic glutamate release, extrasynaptic NMDA receptor activation, and synaptic loss. Proc Natl Acad Sci. 2013;110:2518–2527. - PMC - PubMed
-
- Selkoe DJ, Morris JC, Petersen RC, Lewis J, Davies P, Maloney AJ, Whitehouse PJ, Small DH, Mok SS, Bornstein JC, Davies CA, Mann DM, Sumpter PQ, et al. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. - PubMed
-
- Tajes M, Eraso-Pichot A, Rubio-Moscardó F, Guivernau B, Ramos-Fernández E, Bosch-Morató M, Guix FX, Clarimón J, Miscione GP, Boada M, Gil-Gómez G, Suzuki T, Molina H, et al. Methylglyoxal produced by amyloid-β peptide-induced nitrotyrosination of triosephosphate isomerase triggers neuronal death in Alzheimer's disease. J Alzheimers Dis. 2014;41:273–288. - PubMed
-
- Ramos-Fernández E, Tajes M, Palomer E, Ill-Raga G, Bosch-Morató M, Guivernau B, Román-Dégano I, Eraso-Pichot A, Alcolea D, Fortea J, Nuñez L, Paez A, Alameda F, et al. Posttranslational nitro-glycative modifications of albumin in Alzheimer's disease: implications in cytotoxicity and amyloid-β peptide aggregation. J Alzheimers Dis. 2014;40:643–657. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

