Maintenance of persistent activity in a frontal thalamocortical loop
- PMID: 28467817
- PMCID: PMC6431254
- DOI: 10.1038/nature22324
Maintenance of persistent activity in a frontal thalamocortical loop
Abstract
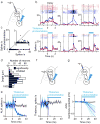
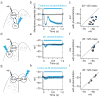
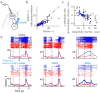
Persistent neural activity maintains information that connects past and future events. Models of persistent activity often invoke reverberations within local cortical circuits, but long-range circuits could also contribute. Neurons in the mouse anterior lateral motor cortex (ALM) have been shown to have selective persistent activity that instructs future actions. The ALM is connected bidirectionally with parts of the thalamus, including the ventral medial and ventral anterior-lateral nuclei. We recorded spikes from the ALM and thalamus during tactile discrimination with a delayed directional response. Here we show that, similar to ALM neurons, thalamic neurons exhibited selective persistent delay activity that predicted movement direction. Unilateral photoinhibition of delay activity in the ALM or thalamus produced contralesional neglect. Photoinhibition of the thalamus caused a short-latency and near-complete collapse of ALM activity. Similarly, photoinhibition of the ALM diminished thalamic activity. Our results show that the thalamus is a circuit hub in motor preparation and suggest that persistent activity requires reciprocal excitation across multiple brain areas.
Figures

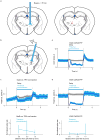
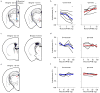


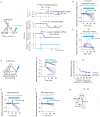



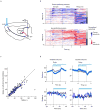



Comment in
-
Working memory: Persistence is key.Nat Rev Neurosci. 2017 Jul;18(7):385. doi: 10.1038/nrn.2017.70. Epub 2017 May 25. Nat Rev Neurosci. 2017. PMID: 28541347 No abstract available.
References
-
- Tanji J, Evarts EV. Anticipatory activity of motor cortex neurons in relation to direction of an intended movement. J Neurophysiol. 1976;39:1062–1068. - PubMed
-
- Murakami M, Vicente MI, Costa GM, Mainen ZF. Neural antecedents of self-initiated actions in secondary motor cortex. Nature neuroscience. 2014;17:1574–1582. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

