Non-equivalence of Wnt and R-spondin ligands during Lgr5+ intestinal stem-cell self-renewal
- PMID: 28467820
- PMCID: PMC5641471
- DOI: 10.1038/nature22313
Non-equivalence of Wnt and R-spondin ligands during Lgr5+ intestinal stem-cell self-renewal
Abstract
The canonical Wnt/β-catenin signalling pathway governs diverse developmental, homeostatic and pathological processes. Palmitoylated Wnt ligands engage cell-surface frizzled (FZD) receptors and LRP5 and LRP6 co-receptors, enabling β-catenin nuclear translocation and TCF/LEF-dependent gene transactivation. Mutations in Wnt downstream signalling components have revealed diverse functions thought to be carried out by Wnt ligands themselves. However, redundancy between the 19 mammalian Wnt proteins and 10 FZD receptors and Wnt hydrophobicity have made it difficult to attribute these functions directly to Wnt ligands. For example, individual mutations in Wnt ligands have not revealed homeostatic phenotypes in the intestinal epithelium-an archetypal canonical, Wnt pathway-dependent, rapidly self-renewing tissue, the regeneration of which is fueled by proliferative crypt Lgr5+ intestinal stem cells (ISCs). R-spondin ligands (RSPO1-RSPO4) engage distinct LGR4-LGR6, RNF43 and ZNRF3 receptor classes, markedly potentiate canonical Wnt/β-catenin signalling, and induce intestinal organoid growth in vitro and Lgr5+ ISCs in vivo. However, the interchangeability, functional cooperation and relative contributions of Wnt versus RSPO ligands to in vivo canonical Wnt signalling and ISC biology remain unknown. Here we identify the functional roles of Wnt and RSPO ligands in the intestinal crypt stem-cell niche. We show that the default fate of Lgr5+ ISCs is to differentiate, unless both RSPO and Wnt ligands are present. However, gain-of-function studies using RSPO ligands and a new non-lipidated Wnt analogue reveal that these ligands have qualitatively distinct, non-interchangeable roles in ISCs. Wnt proteins are unable to induce Lgr5+ ISC self-renewal, but instead confer a basal competency by maintaining RSPO receptor expression that enables RSPO ligands to actively drive and specify the extent of stem-cell expansion. This functionally non-equivalent yet cooperative interaction between Wnt and RSPO ligands establishes a molecular precedent for regulation of mammalian stem cells by distinct priming and self-renewal factors, with broad implications for precise control of tissue regeneration.
Conflict of interest statement
The authors declare competing financial interests. C.Y J., K C.G and C.J.K. are equity shareholders in Surrozen, Inc., which is developing bioengineered Wnt surrogates. G.X.Y.Z., J.M.T., P.B., S.B.Z., H.O., and T.S.M. are equity shareholders in 10x Genomics, Inc., which is developing genomics and single cell transcriptomics technologies.
Figures









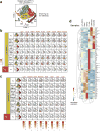
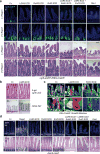
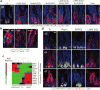
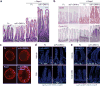


Comment in
-
R-spondin Is More Than Just Wnt's Sidekick.Dev Cell. 2017 Jun 5;41(5):456-458. doi: 10.1016/j.devcel.2017.05.018. Dev Cell. 2017. PMID: 28586642
References
-
- Clevers H, Loh KM, Nusse R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science. 2014;346:1248012. doi:346/6205/1248012 [pii] 10.1126/science.1248012 [doi] - PubMed
-
- Willert K, et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. - PubMed
METHODS REFERENCES
Publication types
MeSH terms
Substances
Grants and funding
- U01 CA176299/CA/NCI NIH HHS/United States
- K08 DK100739/DK/NIDDK NIH HHS/United States
- K99 CA204738/CA/NCI NIH HHS/United States
- K08 DK096048/DK/NIDDK NIH HHS/United States
- U24 DK085532/DK/NIDDK NIH HHS/United States
- U01 DK085527/DK/NIDDK NIH HHS/United States
- U01 DK085547/DK/NIDDK NIH HHS/United States
- R01 GM097015/GM/NIGMS NIH HHS/United States
- R01 DK068326/DK/NIDDK NIH HHS/United States
- U01 DK085532/DK/NIDDK NIH HHS/United States
- U01 CA217851/CA/NCI NIH HHS/United States
- U19 AI116484/AI/NIAID NIH HHS/United States
- P30 CA124435/CA/NCI NIH HHS/United States
- U01 DK085525/DK/NIDDK NIH HHS/United States
- U01 CA151920/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

