Thalamic amplification of cortical connectivity sustains attentional control
- PMID: 28467827
- PMCID: PMC5570520
- DOI: 10.1038/nature22073
Thalamic amplification of cortical connectivity sustains attentional control
Abstract
Although interactions between the thalamus and cortex are critical for cognitive function, the exact contribution of the thalamus to these interactions remains unclear. Recent studies have shown diverse connectivity patterns across the thalamus, but whether this diversity translates to thalamic functions beyond relaying information to or between cortical regions is unknown. Here we show, by investigating the representation of two rules used to guide attention in the mouse prefrontal cortex (PFC), that the mediodorsal thalamus sustains these representations without relaying categorical information. Specifically, mediodorsal input amplifies local PFC connectivity, enabling rule-specific neural sequences to emerge and thereby maintain rule representations. Consistent with this notion, broadly enhancing PFC excitability diminishes rule specificity and behavioural performance, whereas enhancing mediodorsal excitability improves both. Overall, our results define a previously unknown principle in neuroscience; thalamic control of functional cortical connectivity. This function, which is dissociable from categorical information relay, indicates that the thalamus has a much broader role in cognition than previously thought.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

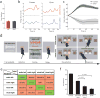




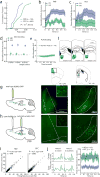
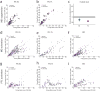




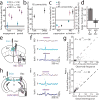

Comment in
-
Working memory: Persistence is key.Nat Rev Neurosci. 2017 Jul;18(7):385. doi: 10.1038/nrn.2017.70. Epub 2017 May 25. Nat Rev Neurosci. 2017. PMID: 28541347 No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

