Dominant protection from HLA-linked autoimmunity by antigen-specific regulatory T cells
- PMID: 28467828
- PMCID: PMC5903850
- DOI: 10.1038/nature22329
Dominant protection from HLA-linked autoimmunity by antigen-specific regulatory T cells
Abstract
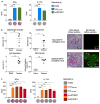
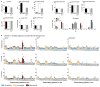
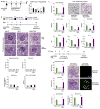
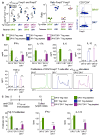
Susceptibility and protection against human autoimmune diseases, including type I diabetes, multiple sclerosis, and Goodpasture disease, is associated with particular human leukocyte antigen (HLA) alleles. However, the mechanisms underpinning such HLA-mediated effects on self-tolerance remain unclear. Here we investigate the molecular mechanism of Goodpasture disease, an HLA-linked autoimmune renal disorder characterized by an immunodominant CD4+ T-cell self-epitope derived from the α3 chain of type IV collagen (α3135-145). While HLA-DR15 confers a markedly increased disease risk, the protective HLA-DR1 allele is dominantly protective in trans with HLA-DR15 (ref. 2). We show that autoreactive α3135-145-specific T cells expand in patients with Goodpasture disease and, in α3135-145-immunized HLA-DR15 transgenic mice, α3135-145-specific T cells infiltrate the kidney and mice develop Goodpasture disease. HLA-DR15 and HLA-DR1 exhibit distinct peptide repertoires and binding preferences and present the α3135-145 epitope in different binding registers. HLA-DR15-α3135-145 tetramer+ T cells in HLA-DR15 transgenic mice exhibit a conventional T-cell phenotype (Tconv) that secretes pro-inflammatory cytokines. In contrast, HLA-DR1-α3135-145 tetramer+ T cells in HLA-DR1 and HLA-DR15/DR1 transgenic mice are predominantly CD4+Foxp3+ regulatory T cells (Treg cells) expressing tolerogenic cytokines. HLA-DR1-induced Treg cells confer resistance to disease in HLA-DR15/DR1 transgenic mice. HLA-DR15+ and HLA-DR1+ healthy human donors display altered α3135-145-specific T-cell antigen receptor usage, HLA-DR15-α3135-145 tetramer+ Foxp3- Tconv and HLA-DR1-α3135-145 tetramer+ Foxp3+CD25hiCD127lo Treg dominant phenotypes. Moreover, patients with Goodpasture disease display a clonally expanded α3135-145-specific CD4+ T-cell repertoire. Accordingly, we provide a mechanistic basis for the dominantly protective effect of HLA in autoimmune disease, whereby HLA polymorphism shapes the relative abundance of self-epitope specific Treg cells that leads to protection or causation of autoimmunity.
Conflict of interest statement
The authors declare no competing interests.
Figures






Comment in
-
Autoimmunity: HLA-mediated protection in Goodpasture disease.Nat Rev Nephrol. 2017 Jul;13(7):381. doi: 10.1038/nrneph.2017.71. Epub 2017 May 15. Nat Rev Nephrol. 2017. PMID: 28502986 No abstract available.
-
Autoimmunity: HLA-mediated protection in Goodpasture disease.Nat Rev Rheumatol. 2017 Jul;13(7):387. doi: 10.1038/nrrheum.2017.76. Epub 2017 May 18. Nat Rev Rheumatol. 2017. PMID: 28515462 No abstract available.
References
-
- Hudson BG, Tryggvason K, Sundaramoorthy M, Neilson EG. Alport’s syndrome, Goodpasture’s syndrome, and type IV collagen. N Engl J Med. 2003;348:2543–2556. - PubMed
-
- Phelps RG, Rees AJ. The HLA complex in Goodpasture’s disease: a model for analyzing susceptibility to autoimmunity. Kidney Int. 1999;56:1638–1653. - PubMed
-
- Cairns LS, et al. The fine specificity and cytokine profile of T-helper cells responsive to the alpha3 chain of type IV collagen in Goodpasture’s disease. J Am Soc Nephrol. 2003;14:2801–2812. - PubMed
-
- Salama AD, et al. Regulation by CD25+ lymphocytes of autoantigen-specific T-cell responses in Goodpasture’s (anti-GBM) disease. Kidney Int. 2003;64:1685–1694. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

