High-Resolution RNA Maps Suggest Common Principles of Splicing and Polyadenylation Regulation by TDP-43
- PMID: 28467899
- PMCID: PMC5437728
- DOI: 10.1016/j.celrep.2017.04.028
High-Resolution RNA Maps Suggest Common Principles of Splicing and Polyadenylation Regulation by TDP-43
Abstract
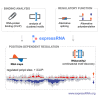
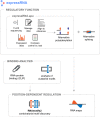
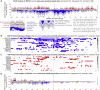
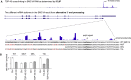
Many RNA-binding proteins (RBPs) regulate both alternative exons and poly(A) site selection. To understand their regulatory principles, we developed expressRNA, a web platform encompassing computational tools for integration of iCLIP and RNA motif analyses with RNA-seq and 3' mRNA sequencing. This reveals at nucleotide resolution the "RNA maps" describing how the RNA binding positions of RBPs relate to their regulatory functions. We use this approach to examine how TDP-43, an RBP involved in several neurodegenerative diseases, binds around its regulated poly(A) sites. Binding close to the poly(A) site generally represses, whereas binding further downstream enhances use of the site, which is similar to TDP-43 binding around regulated exons. Our RNAmotifs2 software also identifies sequence motifs that cluster together with the binding motifs of TDP-43. We conclude that TDP-43 directly regulates diverse types of pre-mRNA processing according to common position-dependent principles.
Keywords: 3′ mRNA sequencing; RNA map; RNAmotifs2; TDP-43; alternative polyadenylation; alternative splicing; clustered sequence motifs; expressRNA; iCLIP; positional regulatory principles.
Copyright © 2017 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Alipanahi B., Delong A., Weirauch M.T., Frey B.J. Predicting the sequence specificities of DNA- and RNA-binding proteins by deep learning. Nat. Biotechnol. 2015;33:831–838. - PubMed
-
- Barash Y., Calarco J.A., Gao W., Pan Q., Wang X., Shai O., Blencowe B.J., Frey B.J. Deciphering the splicing code. Nature. 2010;465:53–59. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

