Calcination Method Synthesis of SnO2/g-C3N4 Composites for a High-Performance Ethanol Gas Sensing Application
- PMID: 28468245
- PMCID: PMC5449979
- DOI: 10.3390/nano7050098
Calcination Method Synthesis of SnO2/g-C3N4 Composites for a High-Performance Ethanol Gas Sensing Application
Abstract
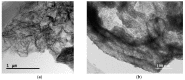
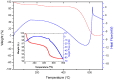
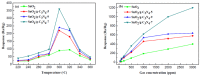
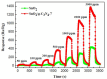
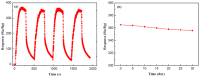
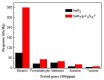
The SnO₂/g-C₃N₄ composites were synthesized via a facile calcination method by using SnCl₄·5H₂O and urea as the precursor. The structure and morphology of the as-synthesized composites were characterized by the techniques of X-ray diffraction (XRD), the field-emission scanning electron microscopy and transmission electron microscopy (FESEM and TEM), energy dispersive spectrometry (EDS), thermal gravity and differential thermal analysis (TG-DTA), and N₂-sorption. The analysis results indicated that the as-synthesized samples possess the two dimensional structure. Additionally, the SnO₂ nanoparticles were highly dispersed on the surface of the g-C₃N₄ nanosheets. The gas-sensing performance of the as-synthesized composites for different gases was tested. Moreover, the composite with 7 wt % g-C₃N₄ content (SnO₂/g-C₃N₄-7) exhibits an admirable gas-sensing property to ethanol, which possesses a higher response and better selectivity than that of the pure SnO2-based sensor. The high surface area of the SnO2/g-C3N4 composite and the good electronic characteristics of the two dimensional graphitic carbon nitride are in favor of the elevated gas-sensing property.
Keywords: SnO2; SnO2/g-C3N4 composite; calcination method; ethanol gas sensing; graphitic carbon nitride.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Hou C., Li J., Huo D., Luo X., Dong J., Yang M., Shi X. A portable embedded toxic gas detection device based on a cross-responsive sensor array. Sens. Actuators B. 2012;161:244–250. doi: 10.1016/j.snb.2011.10.026. - DOI
-
- Ariyageadsakul P., Vchirawongkwin V., Kritayakornupong C. Determination of toxic carbonyl species including acetone, formaldehyde, and phosgene by polyaniline emeraldine gas sensor using DFT calculation. Sens. Actuators B. 2016;232:165–174. doi: 10.1016/j.snb.2016.03.137. - DOI
-
- Hui G.H. Detection of Sulfur Hexafluoride Based on Stochastic Resonance and Miniaturized Sensor Array. Chin. J. Anal. Chem. 2010;38:984–988.
-
- Kaneti Y.V., Yue J., Moriceau J., Chen C., Liu M., Yuan Y., Jiang X., Yu A. Experimental and theoretical studies on noble metal decorated tin oxide flower-like nanorods with high ethanol sensing performance. Sens. Actuators B. 2015;219:83–93. doi: 10.1016/j.snb.2015.04.136. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

