Distinct Expression of Immunoglobulin-Binding Proteins in Shiga Toxin-Producing Escherichia coli Implicates High Protein Stability and a Characteristic Phenotype
- PMID: 28468281
- PMCID: PMC5450701
- DOI: 10.3390/toxins9050153
Distinct Expression of Immunoglobulin-Binding Proteins in Shiga Toxin-Producing Escherichia coli Implicates High Protein Stability and a Characteristic Phenotype
Abstract
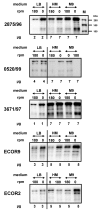
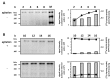
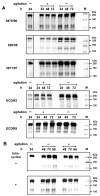
Several immunoglobulin-binding proteins of Escherichia coli (Eib) have been isolated from both non-pathogenic and pathogenic E. coli strains. Shiga toxin (Stx)-producing E. coli (STEC) contain eibG either as a single gene or in combination with eibC, while other E. coli strains harbour single or multiple eib genes. The Eib proteins bind human immunoglobulins in a non-immune manner and contribute to bacterial chain-like adherence to human epithelial cells. In this study, the EibG expression in several STEC strains was analysed under different environmental conditions. STEC produced high levels of EibG in complex media and lower levels in low-grade and minimal media under static growth conditions. This characteristic was independent on the Eib subtypes. Microscopically, EibG-expressing STEC exhibited chain formation and aggregation in all employed media, while aggregates were only visible after growth in complex medium. Once expressed, EibG proteins demonstrate high stability during prolonged incubation. Our findings indicate that the regulation of the expression of Eib proteins is highly complex, although the protein levels vary among STEC strains. However, positive upregulation conditions generally result in distinct phenotypes of the isolates.
Keywords: Shiga toxin-producing Escherichia coli; expression; immunoglobulin-binding protein G; regulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Agitation down-regulates immunoglobulin binding protein EibG expression in Shiga toxin-producing Escherichia coli (STEC).PLoS One. 2015 Mar 6;10(3):e0119583. doi: 10.1371/journal.pone.0119583. eCollection 2015. PLoS One. 2015. PMID: 25746924 Free PMC article.
-
A new immunoglobulin-binding protein, EibG, is responsible for the chain-like adhesion phenotype of locus of enterocyte effacement-negative, shiga toxin-producing Escherichia coli.Infect Immun. 2006 Oct;74(10):5747-55. doi: 10.1128/IAI.00724-06. Infect Immun. 2006. PMID: 16988252 Free PMC article.
-
Distribution and phylogeny of immunoglobulin-binding protein G in Shiga toxin-producing Escherichia coli and its association with adherence phenotypes.Infect Immun. 2010 Aug;78(8):3625-36. doi: 10.1128/IAI.00006-10. Epub 2010 Jun 14. Infect Immun. 2010. PMID: 20547747 Free PMC article.
-
Virulence characteristics of Shiga toxin-producing Escherichia coli from raw meats and clinical samples.Microbiol Immunol. 2015 Mar;59(3):114-22. doi: 10.1111/1348-0421.12235. Microbiol Immunol. 2015. PMID: 25644201
-
Virulence genes, Shiga toxin subtypes, major O-serogroups, and phylogenetic background of Shiga toxin-producing Escherichia coli strains isolated from cattle in Iran.Microb Pathog. 2017 Aug;109:274-279. doi: 10.1016/j.micpath.2017.05.041. Epub 2017 May 31. Microb Pathog. 2017. PMID: 28578089
Cited by
-
Description and Comparative Genomics of Macrococcus caseolyticus subsp. hominis subsp. nov., Macrococcus goetzii sp. nov., Macrococcus epidermidis sp. nov., and Macrococcus bohemicus sp. nov., Novel Macrococci From Human Clinical Material With Virulence Potential and Suspected Uptake of Foreign DNA by Natural Transformation.Front Microbiol. 2018 Jun 13;9:1178. doi: 10.3389/fmicb.2018.01178. eCollection 2018. Front Microbiol. 2018. PMID: 29951040 Free PMC article.
-
Shiga Toxin-Producing Escherichia coli and Milk Fat Globules.Microorganisms. 2022 Feb 23;10(3):496. doi: 10.3390/microorganisms10030496. Microorganisms. 2022. PMID: 35336072 Free PMC article. Review.
-
Current Promising Strategies against Antibiotic-Resistant Bacterial Infections.Antibiotics (Basel). 2022 Dec 30;12(1):67. doi: 10.3390/antibiotics12010067. Antibiotics (Basel). 2022. PMID: 36671268 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

