Heparin, Heparan Sulphate and the TGF-β Cytokine Superfamily
- PMID: 28468283
- PMCID: PMC6154108
- DOI: 10.3390/molecules22050713
Heparin, Heparan Sulphate and the TGF-β Cytokine Superfamily
Abstract
Of the circa 40 cytokines of the TGF-β superfamily, around a third are currently known to bind to heparin and heparan sulphate. This includes TGF-β1, TGF-β2, certain bone morphogenetic proteins (BMPs) and growth and differentiation factors (GDFs), as well as GDNF and two of its close homologues. Experimental studies of their heparin/HS binding sites reveal a diversity of locations around the shared cystine-knot protein fold. The activities of the TGF-β cytokines in controlling proliferation, differentiation and survival in a range of cell types are in part regulated by a number of specific, secreted BMP antagonist proteins. These vary in structure but seven belong to the CAN or DAN family, which shares the TGF-β type cystine-knot domain. Other antagonists are more distant members of the TGF-β superfamily. It is emerging that the majority, but not all, of the antagonists are also heparin binding proteins. Any future exploitation of the TGF-β cytokines in the therapy of chronic diseases will need to fully consider their interactions with glycosaminoglycans and the implications of this in terms of their bioavailability and biological activity.
Keywords: BMP antagonists; GDNF; TGF-β; bone morphogenetic protein (BMP); gremlin; growth and differentiation factor (GDF); heparan sulphate; heparin; noggin; sclerostin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


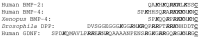

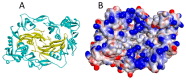
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

