Association of Extracellular Membrane Vesicles with Cutaneous Wound Healing
- PMID: 28468315
- PMCID: PMC5454869
- DOI: 10.3390/ijms18050956
Association of Extracellular Membrane Vesicles with Cutaneous Wound Healing
Abstract
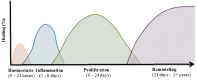
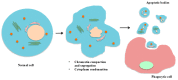
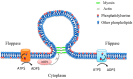
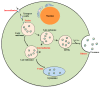
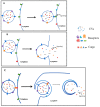
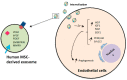
Extracellular vesicles (EVs) are membrane-enclosed vesicles that are released into the extracellular environment by various cell types, which can be classified as apoptotic bodies, microvesicles and exosomes. EVs have been shown to carry DNA, small RNAs, proteins and membrane lipids which are derived from the parental cells. Recently, several studies have demonstrated that EVs can regulate many biological processes, such as cancer progression, the immune response, cell proliferation, cell migration and blood vessel tube formation. This regulation is achieved through the release and transport of EVs and the transfer of their parental cell-derived molecular cargo to recipient cells. This thereby influences various physiological and sometimes pathological functions within the target cells. While intensive investigation of EVs has focused on pathological processes, the involvement of EVs in normal wound healing is less clear; however, recent preliminarily investigations have produced some initial insights. This review will provide an overview of EVs and discuss the current literature regarding the role of EVs in wound healing, especially, their influence on coagulation, cell proliferation, migration, angiogenesis, collagen production and extracellular matrix remodelling.
Keywords: angiogenesis; apoptotic bodies; endothelial cells; exosomes; extracellular membrane vesicles; keratinocytes; microvesicles; migration; proliferation; wound healing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Hao S., Bai O., Yuan J., Qureshi M., Xiang J. Dendritic cell-derived exosomes stimulate stronger CD8+ CTL responses and antitumor immunity than tumor cell-derived exosomes. Cell. Mol. Immunol. 2006;3:205–211. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

