Buprenorphine for the Treatment of the Neonatal Abstinence Syndrome
- PMID: 28468518
- PMCID: PMC5662132
- DOI: 10.1056/NEJMoa1614835
Buprenorphine for the Treatment of the Neonatal Abstinence Syndrome
Abstract
Background: Current pharmacologic treatment of the neonatal abstinence syndrome with morphine is associated with a lengthy duration of therapy and hospitalization. Buprenorphine may be more effective than morphine for this indication.
Methods: In this single-site, double-blind, double-dummy clinical trial, we randomly assigned 63 term infants (≥37 weeks of gestation) who had been exposed to opioids in utero and who had signs of the neonatal abstinence syndrome to receive either sublingual buprenorphine or oral morphine. Infants with symptoms that were not controlled with the maximum dose of opioid were treated with adjunctive phenobarbital. The primary end point was the duration of treatment for symptoms of neonatal opioid withdrawal. Secondary clinical end points were the length of hospital stay, the percentage of infants who required supplemental treatment with phenobarbital, and safety.
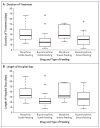
Results: The median duration of treatment was significantly shorter with buprenorphine than with morphine (15 days vs. 28 days), as was the median length of hospital stay (21 days vs. 33 days) (P<0.001 for both comparisons). Adjunctive phenobarbital was administered in 5 of 33 infants (15%) in the buprenorphine group and in 7 of 30 infants (23%) in the morphine group (P=0.36). Rates of adverse events were similar in the two groups.
Conclusions: Among infants with the neonatal abstinence syndrome, treatment with sublingual buprenorphine resulted in a shorter duration of treatment and shorter length of hospital stay than treatment with oral morphine, with similar rates of adverse events. (Funded by the National Institute on Drug Abuse; BBORN ClinicalTrials.gov number, NCT01452789 .).
Figures
Comment in
-
Buprenorphine for the Neonatal Abstinence Syndrome.N Engl J Med. 2017 Sep 7;377(10):996. doi: 10.1056/NEJMc1709121. N Engl J Med. 2017. PMID: 28877015 No abstract available.
-
Buprenorphine for the Neonatal Abstinence Syndrome.N Engl J Med. 2017 Sep 7;377(10):996. doi: 10.1056/NEJMc1709121. N Engl J Med. 2017. PMID: 28880502 No abstract available.
-
Buprenorphine for the Neonatal Abstinence Syndrome.N Engl J Med. 2017 Sep 7;377(10):996-7. doi: 10.1056/NEJMc1709121. N Engl J Med. 2017. PMID: 28880503 No abstract available.
References
-
- Hudak ML, Tan RC. Neonatal drug withdrawal. Pediatrics. 2012;129(2):e540–e560. - PubMed
-
- Holmes AV, Atwood EC, Whalen B, et al. Rooming-in to treat neonatal abstinence syndrome: improved family-centered care at lower cost. Pediatrics. 2016;137(6):e20152929. - PubMed
-
- Short VL, Gannon M, Abatemarco DJ. The association between breastfeeding and length of hospital stay among infants diagnosed with neonatal abstinence syndrome: a population-based study of in-hospital births. Breastfeed Med. 2016;11:343–9. - PubMed
-
- Osborn DA, Jeffery HE, Cole MJ. Opiate treatment for opiate withdrawal in newborn infants. Cochrane Database Syst Rev. 2010:CD002059. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
