Cell-penetrating peptide conjugates to enhance the antitumor effect of paclitaxel on drug-resistant lung cancer
- PMID: 28468542
- PMCID: PMC8253140
- DOI: 10.1080/10717544.2017.1321060
Cell-penetrating peptide conjugates to enhance the antitumor effect of paclitaxel on drug-resistant lung cancer
Abstract
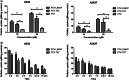
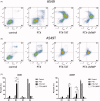
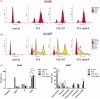
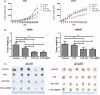
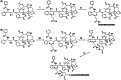
To conquer the drug resistance of tumors and the poor solubility of paclitaxel (PTX), two PTX-cell-penetrating peptide conjugates (PTX-CPPs), PTX-TAT and PTX-LMWP, were synthesized and evaluated for the first time. Compared with free PTX, PTX-CPPs displayed significantly enhanced cellular uptake, elevated cell toxicity, increased cell apoptosis, and decreased mitochondrial membrane potential (Δψm) in both A549 and A549T cells. PTX-LMWP exhibited a stronger inhibitory effect than PTX-TAT in A549T cells. Analysis of cell-cycle distribution showed that PTX-LMWP influenced mitosis in drug-resistant A549T tumor cells via a different mechanism than PTX. PTX-CPPs were more efficient in inhibiting tumor growth in tumor-bearing mice than free PTX, which suggested their better in vivo antitumor efficacy. Hence, this study demonstrates that PTX-CPPs, particularly PTX-LMWP, have outstanding potential for inhibiting the growth of tumors and are a promising approach for treating lung cancer, especially drug-resistant lung cancer.
Keywords: Paclitaxel; TAT; cell-penetrating peptide; conjugate; drug-resistant lung cancer; low molecular weight protamine.
Conflict of interest statement
The authors declare no competing financial interests. The authors thank the National Basic Research Program of China (No. 2013CB 932500), the National Natural Science Foundation of China (No. 81361140344), and the Development Project of Shanghai Peak Disciplines-Integrated Medicine (No. 20150407) for financial support.
Figures


References
-
- Chen Z, Zhang P, Cheetham AG, et al. . (2014). Controlled release of free doxorubicin from peptide-drug conjugates by drug loading. J Control Release 191:123–30 - PubMed
-
- Chi KN, Higano C, Reeves J, et al. . (2014). A randomized phase III study comparing first line docetaxel and prednisone (DP) to DP plus custirsen in men with metastatic castration resistant prostate cancer. Ann Oncol 25:255–79
-
- Christie RJ, Findley DJ, Grainger DW. (2004). Design and synthesis of a new polymer drug delivery conjugate. Biomed Sci Instrum 40:136–41 - PubMed
-
- De Hoon JP, Veeck J, Vriens BE, et al. . (2012). Taxane resistance in breast cancer: a closed HER2 circuit? Biochim Biophys Acta 1825:197–206 - PubMed
-
- De La Torre BG, Hornillos V, Luque-Ortega JR, et al. . (2014). A BODIPY-embedding miltefosine analog linked to cell-penetrating Tat(48–60) peptide favors intracellular delivery and visualization of the antiparasitic drug. Amino Acids 46:1047–58 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
