Drug-transporter mediated interactions between anthelminthic and antiretroviral drugs across the Caco-2 cell monolayers
- PMID: 28468637
- PMCID: PMC5415745
- DOI: 10.1186/s40360-017-0129-6
Drug-transporter mediated interactions between anthelminthic and antiretroviral drugs across the Caco-2 cell monolayers
Abstract
Background: Drug interactions between antiretroviral drugs (ARVs) and anthelminthic drugs, ivermectin (IVM) and praziquantel (PZQ) were assessed by investigating their permeation through the Caco-2 cell monolayers in a transwell. The impact of anthelminthics on the transport of ARVs was determined by assessing the apical to basolateral (AP → BL) [passive] and basolateral to apical (BL → AP) [efflux] directions alone, and in presence of an anthelminthic. The reverse was conducted for the assessment of the influence of ARVs on anthelminthics.
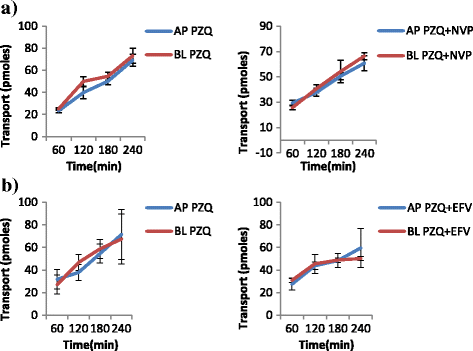
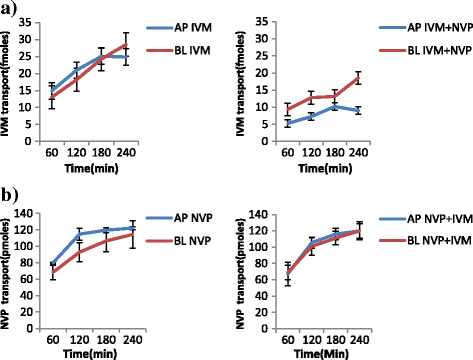
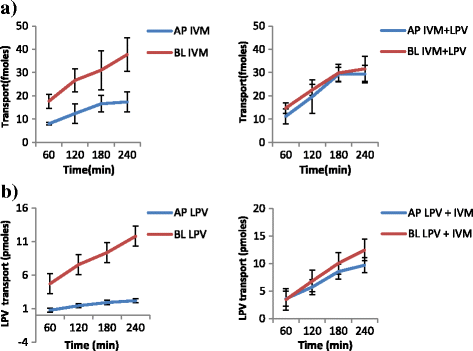
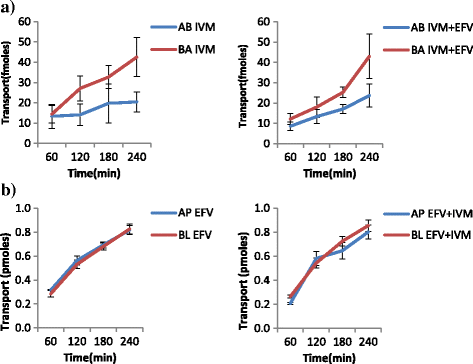
Methods: Samples from the AP and BL compartments were taken at 60, 120, 180 and 240 min and quantified either by HPLC or radiolabeled assay using a liquid scintillating counter for the respective drugs. Transepithelial resistance (TEER) was used to assess the integrity of the monolayers. The amount of compound transported per second (apparent permeability, Papp) was calculated for both AP to BL (PappAtoB), and BL to AP (PappBtoA) movements. Samples collected after 60 min were used to determine the efflux ratio (ER), quotient of secretory permeability and absorptive permeability (PappBL-AP/PappAP-BL). The reverse, (PappAP-BL/PappBL-AP) constituted the uptake ratio. The impact of SQV, EFV and NVP on the transport of both IVM and PZQ were investigated. The effect of LPV on the transport of IVM was also determined. The influence of IVM on the transport of SQV, NVP, LPV and EFV; as well as the effect PZQ on the transport of SQV of was also investigated, and a two-tailed p value of <0.05 was considered significant.
Results: IVM significantly inhibited the efflux transport (BL → AP movement) of LPV (ER; 6.7 vs. 0.8, p = 0.0038) and SQV (ER; 3.1 vs. 1.2 p = 0.00328); and increased the efflux transport of EFV (ER; 0.7 vs. 0.9, p = 0.031) suggesting the possibility of drug transporter mediated interactions between the two drugs. NVP increased the efflux transport of IVM (ER; 0.8 vs. 1.8, p = 0.0094).
Conclusions: The study provides in vitro evidence of potential interactions between IVM, an anthelminthic drug with antiretroviral drugs; LPV, SQV, NVP and EFV. Further investigations should be conducted to investigate the possibility of in vivo interactions.
Keywords: Antiparasitic; Antiretroviral; Caco-2 cell monolayers; Drug interactions; Drug transport; Intestinal epithelium; TEER.
Figures



References
-
- Seden K, Khoo S, Back D, Prevatt N, Lamorde M, Byakika-Kibwika P, Mayito J, Ryan M, Merry C. Drug-drug interactions between antiretrovirals and drugs used in the management of neglected tropical diseases: important considerations in the WHO 2020 Roadmap and London Declaration on Neglected Tropical Diseases. AIDS (London, England) 2013;27:675–-686. doi: 10.1097/QAD.0b013e32835ca9b4. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

