ICAM-1 is a key receptor mediating cytoadherence and pathology in the Plasmodium chabaudi malaria model
- PMID: 28468674
- PMCID: PMC5415785
- DOI: 10.1186/s12936-017-1834-8
ICAM-1 is a key receptor mediating cytoadherence and pathology in the Plasmodium chabaudi malaria model
Abstract
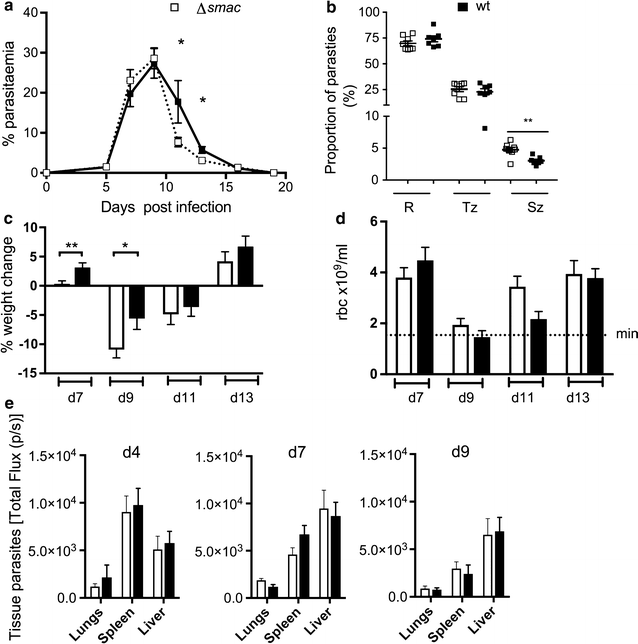
Background: Parasite cytoadherence within the microvasculature of tissues and organs of infected individuals is implicated in the pathogenesis of several malaria syndromes. Multiple host receptors may mediate sequestration. The identity of the host receptor(s), or the parasite ligand(s) responsible for sequestration of Plasmodium species other than Plasmodium falciparum is largely unknown. The rodent malaria parasites may be useful to model interactions of parasite species, which lack the var genes with their respective hosts, as other multigene families are shared between the species. The role of the endothelial receptors ICAM-1 and CD36 in cytoadherence and in the development of pathology was investigated in a Plasmodium chabaudi infection in C57BL/6 mice lacking these receptors. The schizont membrane-associated cytoadherence (SMAC) protein of Plasmodium berghei has been shown to exhibit reduced CD36-associated cytoadherence in P. berghei ANKA-infected mice.
Methods: Parasite tissue sequestration and the development of acute stage pathology in P. chabaudi infections of mice lacking CD36 or ICAM-1, their respective wild type controls, and in infections with mutant P. chabaudi parasites lacking the smac gene were compared. Peripheral blood parasitaemia, red blood cell numbers and weight change were monitored throughout the courses of infection. Imaging of bioluminescent parasites in isolated tissues (spleen, lungs, liver, kidney and gut) was used to measure tissue parasite load.
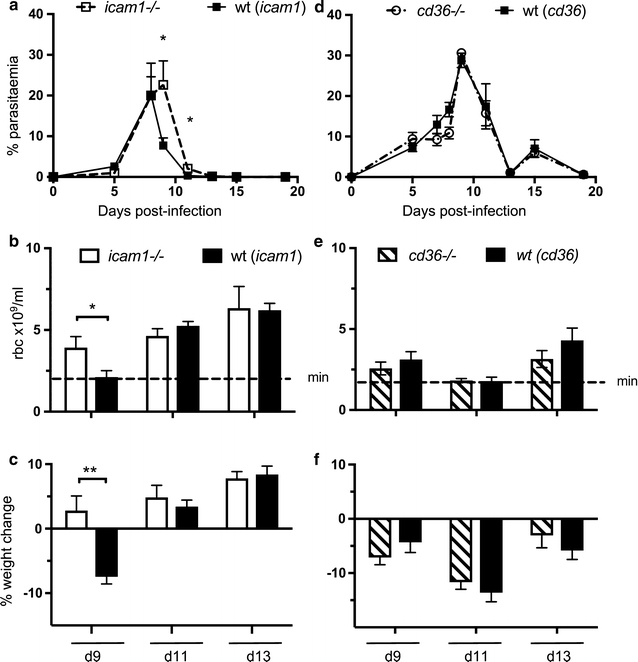
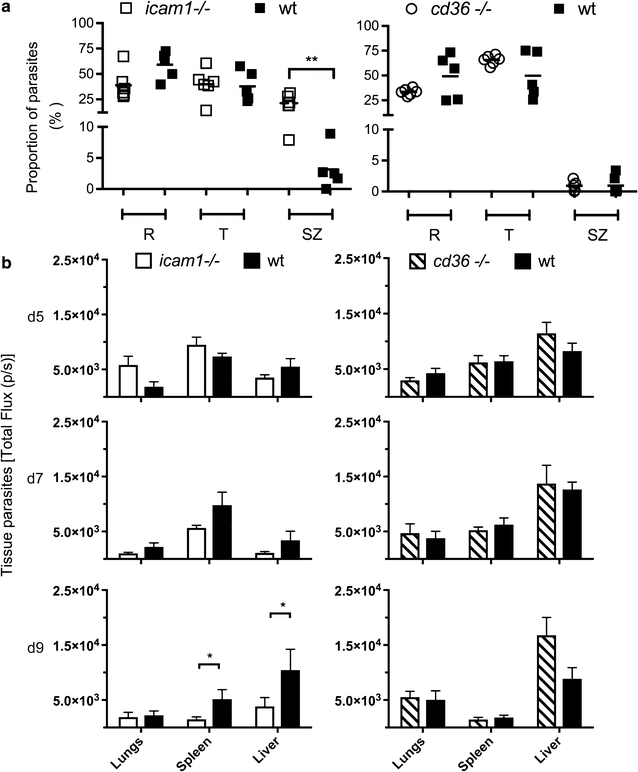
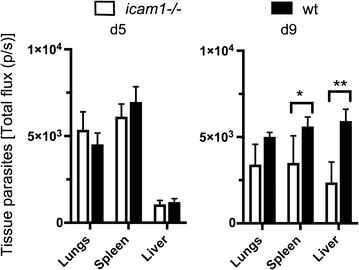
Results: This study shows that neither the lack of CD36 nor the deletion of the smac gene from P. chabaudi significantly impacted on acute-stage pathology or parasite sequestration. By contrast, in the absence of ICAM-1, infected animals experience less anaemia and weight loss, reduced parasite accumulation in both spleen and liver and higher peripheral blood parasitaemia during acute stage malaria. The reduction in parasite tissue sequestration in infections of ICAM-1 null mice is maintained after mosquito transmission.
Conclusions: These results indicate that ICAM-1-mediated cytoadherence is important in the P. chabaudi model of malaria and suggest that for rodent malarias, as for P. falciparum, there may be multiple host and parasite molecules involved in sequestration.
Keywords: CD36; ICAM-1; Plasmodium chabaudi; SMAC; Schizont membrane-associated cytoadherence protein; Sequestration.
Figures
Similar articles
-
Reduced CD36-dependent tissue sequestration of Plasmodium-infected erythrocytes is detrimental to malaria parasite growth in vivo.J Exp Med. 2012 Jan 16;209(1):93-107. doi: 10.1084/jem.20110762. Epub 2011 Dec 19. J Exp Med. 2012. PMID: 22184632 Free PMC article.
-
Characterization and tissue-specific expression patterns of the Plasmodium chabaudi cir multigene family.Malar J. 2011 Sep 19;10:272. doi: 10.1186/1475-2875-10-272. Malar J. 2011. PMID: 21929749 Free PMC article.
-
Plasmodium chabaudi-infected erythrocytes adhere to CD36 and bind to microvascular endothelial cells in an organ-specific way.Infect Immun. 2000 Jul;68(7):4135-44. doi: 10.1128/IAI.68.7.4135-4144.2000. Infect Immun. 2000. PMID: 10858230 Free PMC article.
-
Sequestration and tissue accumulation of human malaria parasites: can we learn anything from rodent models of malaria?PLoS Pathog. 2010 Sep 30;6(9):e1001032. doi: 10.1371/journal.ppat.1001032. PLoS Pathog. 2010. PMID: 20941396 Free PMC article. Review.
-
Cytoadherence and sequestration in Plasmodium falciparum: defining the ties that bind.Microbes Infect. 2003 Aug;5(10):897-909. doi: 10.1016/s1286-4579(03)00162-x. Microbes Infect. 2003. PMID: 12919858 Review.
Cited by
-
Attenuated T Cell Responses Are Associated With the Blockade of Cerebral Malaria Development by YOP1-Deficient Plasmodium berghei ANKA.Front Immunol. 2021 May 6;12:642585. doi: 10.3389/fimmu.2021.642585. eCollection 2021. Front Immunol. 2021. PMID: 34025654 Free PMC article.
-
Comparative proteomics reveals Cryptosporidium parvum manipulation of the host cell molecular expression and immune response.PLoS Negl Trop Dis. 2021 Nov 24;15(11):e0009949. doi: 10.1371/journal.pntd.0009949. eCollection 2021 Nov. PLoS Negl Trop Dis. 2021. PMID: 34818332 Free PMC article.
-
Host-Derived Extracellular Vesicles in Blood and Tissue Human Protozoan Infections.Microorganisms. 2023 Sep 14;11(9):2318. doi: 10.3390/microorganisms11092318. Microorganisms. 2023. PMID: 37764162 Free PMC article. Review.
-
Cerebral Malaria Model Applying Human Brain Organoids.Cells. 2023 Mar 23;12(7):984. doi: 10.3390/cells12070984. Cells. 2023. PMID: 37048057 Free PMC article.
-
G6pd-Deficient Mice Are Protected From Experimental Cerebral Malaria and Liver Injury by Suppressing Proinflammatory Response in the Early Stage of Plasmodium berghei Infection.Front Immunol. 2021 Aug 11;12:719189. doi: 10.3389/fimmu.2021.719189. eCollection 2021. Front Immunol. 2021. PMID: 34456927 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

