CD3+CD4negCD8neg (double negative) T lymphocytes and NKT cells as the main cytotoxic-related-CD107a+ cells in lesions of cutaneous leishmaniasis caused by Leishmania (Viannia) braziliensis
- PMID: 28468680
- PMCID: PMC5415843
- DOI: 10.1186/s13071-017-2152-2
CD3+CD4negCD8neg (double negative) T lymphocytes and NKT cells as the main cytotoxic-related-CD107a+ cells in lesions of cutaneous leishmaniasis caused by Leishmania (Viannia) braziliensis
Abstract
Background: Cutaneous leishmaniasis (CL) is caused by Leishmania (Viannia) braziliensis, which infects dermal macrophages and dendritic cells, causing an intense immune-mediated-tissue inflammation and a skin ulcer with elevated borders that can heal spontaneously or after antimonial therapy. The resolution of lesions depends on an adaptive immune response, and cytotoxic cells seem to have a fundamental role in this process. The aim of this study is to better understand the role of cytotoxicity mediated mechanisms that occur during the immune response in the CL lesion milieu, considering distinct cytotoxic-related CD107a+ cells, such as CD8+, CD4+, CD4neg CD8neg (double-negative, DN) and CD4+CD8+ (double-positive, DP) T lymphocytes, as well as NK and NKT cells.
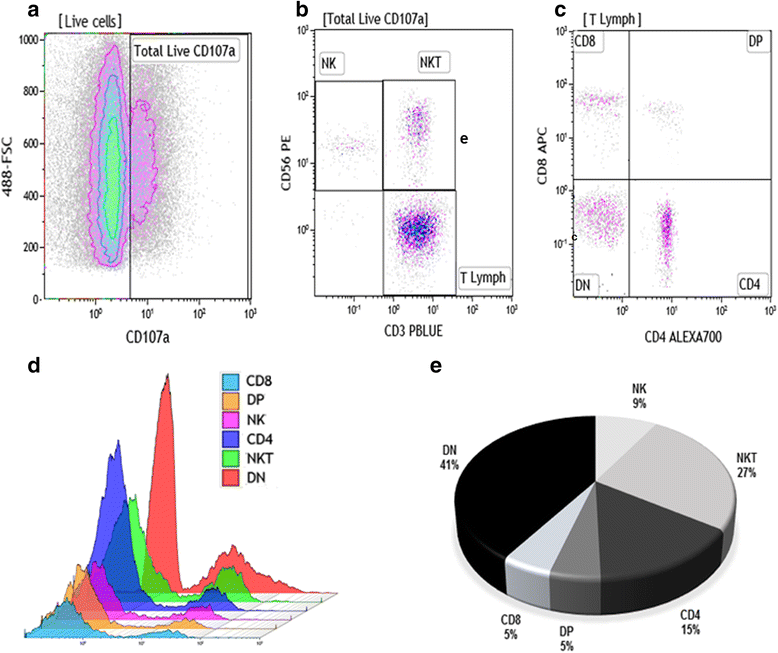
Methods: Lesion derived cells were assessed for T cell subpopulations and NK cells, as well as CD107a expression by flow cytometry. In addition, cytometric bead array (CBA) was used to quantify cytokines and granzyme B concentrations in supernatants from macerated lesions.
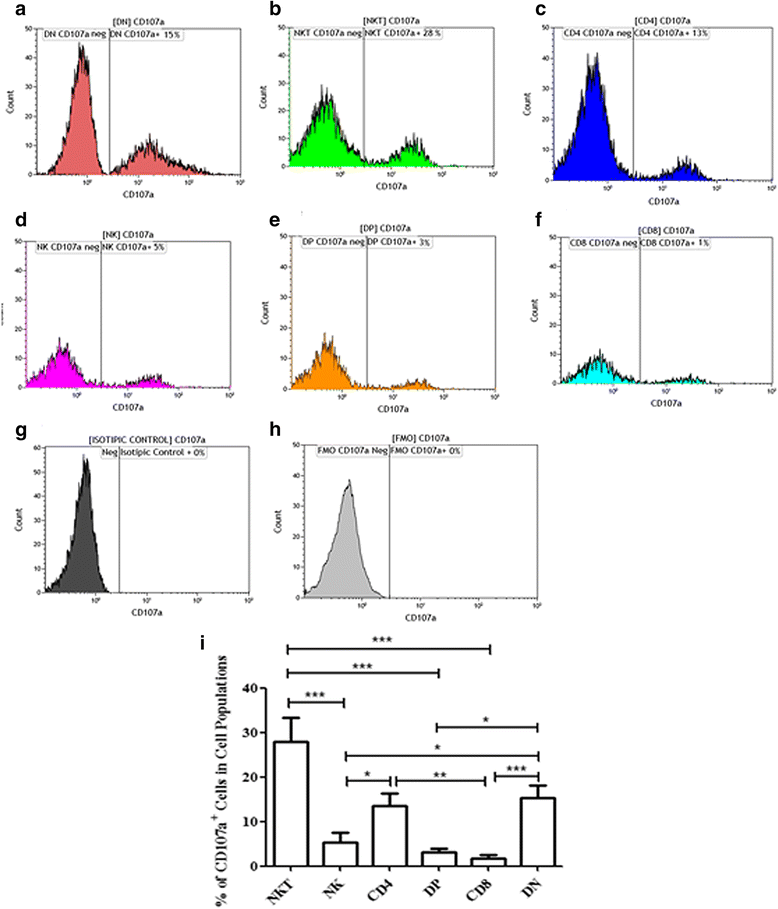
Results: Flow cytometry analyses revealed that NKT cells are the major CD107a-expressing cell population committed to cytotoxicity in CL lesion, although we also observed high frequencies of CD4+ and DN T cells expressing CD107a. Analysing the pool of CD107a+-cell populations, we found a higher distribution of DN T cells (44%), followed by approximately 25% of NKT cells. Interestingly, NK and CD8+ T cells represented only 3 and 4% of the total-CD107a+-cell pool, respectively.
Conclusions: The cytotoxicity activity that occurs in the lesion milieu of CL patients seems to be dominated by DN T and NKT cells. These findings suggest the need for a reevaluation of the role of classical-cytotoxic NK and CD8+ T cells in the pathogenesis of CL, implicating an important role for other T cell subpopulations.
Keywords: CD107a; Cytotoxicity; Double-negative T lymphocytes; Flow cytometry; Human cutaneous leishmaniasis; Leishmania (Viannia) braziliensis; Lesion; NKT cells.
Figures


References
-
- WHO | Leishmaniasis. WHO. [cited 2015 Mar 25]. Available from: http://www.who.int/mediacentre/factsheets/fs375/en/
-
- Brelaz-de-Castro MCA, de Almeida AF, de Oliveira AP, de Assis-Souza M, da Rocha LF, Pereira VRA. Cellular immune response evaluation of cutaneous leishmaniasis patient cells stimulated with Leishmania (Viannia) braziliensis antigenic fractions before and after clinical cure. Cell Immunol. 2012;279:180–6. doi: 10.1016/j.cellimm.2012.11.006. - DOI - PubMed
-
- Bottrel RL, Dutra WO, Martins FA, Gontijo B, Carvalho E, Barral-Netto M, et al. Flow cytometric determination of cellular sources and frequencies of key cytokine-producing lymphocytes directed against recombinant LACK and soluble Leishmania antigen in human cutaneous leishmaniasis. Infect Immun. 2001;69:3232–9. doi: 10.1128/IAI.69.5.3232-3239.2001. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

