Risk of Intussusception After Rotavirus Vaccination
- PMID: 28468712
- PMCID: PMC5424085
- DOI: 10.3238/arztebl.2017.0255
Risk of Intussusception After Rotavirus Vaccination
Abstract
Background: In 2013, the German Standing Committee on Vaccination (Ständige Impfkommission, STIKO) recommended rotavirus (RV) vaccination for all infants while stating that this mildly increased the risk of intussusception, a potentially life-threatening event. Since this recommendation was issued, multiple observational studies on this topic designed as self-controlled case series (SCCS) have been published. The SCCS design is particularly suitable for the study of rare adverse effects of medications.
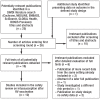
Methods: We systematically searched the literature for SCCS studies on the risk of intussusception after RV vaccination. Relative risks (RR) corresponding to different doses of vaccine were summarized in a meta-analysis, and attributable risks (AR) were calculated.
Results: Of the 16 initially identified studies, 10 with a predominantly low risk of bias were considered in the analysis. The RR for intussusception was 5.71 (95% confidence interval [4.50; 7.25]) 1-7 days after the first dose, 1.69 [1.33; 2.14] after the second, and 1.14 [0.75; 1.74] after the third. The AR for children of the age at which RV vaccination is recommended was 1.7 [1.1; 2.7] additional intussusceptions per 100 000 vaccinated children after the first dose and 0.25 [0.16; 0.40] after the second. If >3-month-old infants are vaccinated, the AR is higher: 5.6 [4.3; 7.2] per 100 000 after the first dose and 0.81 [0.63; 1.06] per 100 000 after the second.
Conclusion: RV vaccination is associated with a markedly elevated RR and a mildly elevated AR for intussusception 1-7 days after the first dose. Physicians should begin the series of vaccinations at age 6-12 weeks, as recommended by the STIKO, because the risk of intussusception is higher afterward. Current health insurance company claim data indicate that 11.2% of infants are still receiving the first dose of the vaccine at ages above 3 months. The parents of vaccinated children should be informed about the possible signs of intussusception (colicky pain, bilious vomiting, and red "currant jelly" stool).
Figures





Similar articles
-
Association Between Rotavirus Vaccination and Risk of Intussusception Among Neonates and Infants: A Systematic Review and Meta-analysis.JAMA Netw Open. 2019 Oct 2;2(10):e1912458. doi: 10.1001/jamanetworkopen.2019.12458. JAMA Netw Open. 2019. PMID: 31584679 Free PMC article.
-
Risk of intussusception after monovalent rotavirus vaccination.N Engl J Med. 2014 Feb 6;370(6):513-9. doi: 10.1056/NEJMoa1311738. Epub 2014 Jan 14. N Engl J Med. 2014. PMID: 24422678
-
The risk of intussusception following monovalent rotavirus vaccination in England: A self-controlled case-series evaluation.Vaccine. 2016 Jul 12;34(32):3684-9. doi: 10.1016/j.vaccine.2016.04.050. Epub 2016 Jun 7. Vaccine. 2016. PMID: 27286641
-
Background paper to the recommendation for routine rotavirus vaccination of infants in Germany.Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2013 Jul;56(7):957-84. doi: 10.1007/s00103-013-1777-3. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2013. PMID: 23807405
-
Rotavirus live, oral, pentavalent vaccine.Clin Ther. 2007 Dec;29(12):2724-37. doi: 10.1016/j.clinthera.2007.12.018. Clin Ther. 2007. PMID: 18201590 Review.
Cited by
-
National stakeholder preferences for next-generation rotavirus vaccines: Results from a six-country study.Vaccine. 2022 Jan 21;40(2):370-379. doi: 10.1016/j.vaccine.2021.11.009. Epub 2021 Dec 2. Vaccine. 2022. PMID: 34863614 Free PMC article.
-
Clinical Characteristics of Intussusception with Surgical Reduction: a Single-Center Experience with 568 Cases.J Gastrointest Surg. 2019 Nov;23(11):2255-2262. doi: 10.1007/s11605-019-04178-0. Epub 2019 Mar 11. J Gastrointest Surg. 2019. PMID: 30859429
-
The Apparent Lack of the Risk of Intussusception Immediately After Rotavirus Vaccination Among Japanese Infants.Viruses. 2024 Nov 10;16(11):1758. doi: 10.3390/v16111758. Viruses. 2024. PMID: 39599872 Free PMC article.
-
Experience of establishing and coordinating a nationwide network for bidirectional intussusception surveillance in India: lessons for multisite research studies.BMJ Open. 2021 May 28;11(5):e046827. doi: 10.1136/bmjopen-2020-046827. BMJ Open. 2021. PMID: 34049918 Free PMC article.
-
In Vitro Evaluation of Anti-Rotaviral Activity and Intestinal Toxicity of a Phytotherapeutic Prototype of Achyrocline bogotensis (Kunth) DC.Viruses. 2022 Oct 29;14(11):2394. doi: 10.3390/v14112394. Viruses. 2022. PMID: 36366492 Free PMC article.
References
-
- Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:136–141. - PubMed
-
- Koch J, Wiese-Posselt M. Epidemiology of rotavirus infections in children less than 5 years of age: Germany, 2001-2008. Pediatr Infect Dis J. 2011;30:112–117. - PubMed
-
- European Medicines Agency. Rotarix. Scientific discussion, 11 September 2006. www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussi... (last accessed on 30 March 2012)
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

