A voltage-dependent K+ channel in the lysosome is required for refilling lysosomal Ca2+ stores
- PMID: 28468834
- PMCID: PMC5461029
- DOI: 10.1083/jcb.201612123
A voltage-dependent K+ channel in the lysosome is required for refilling lysosomal Ca2+ stores
Abstract
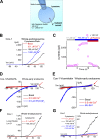
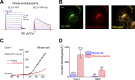
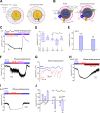
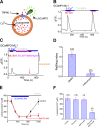
The resting membrane potential (Δψ) of the cell is negative on the cytosolic side and determined primarily by the plasma membrane's selective permeability to K+ We show that lysosomal Δψ is set by lysosomal membrane permeabilities to Na+ and H+, but not K+, and is positive on the cytosolic side. An increase in juxta-lysosomal Ca2+ rapidly reversed lysosomal Δψ by activating a large voltage-dependent and K+-selective conductance (LysoKVCa). LysoKVCa is encoded molecularly by SLO1 proteins known for forming plasma membrane BK channels. Opening of single LysoKVCa channels is sufficient to cause the rapid, striking changes in lysosomal Δψ. Lysosomal Ca2+ stores may be refilled from endoplasmic reticulum (ER) Ca2+ via ER-lysosome membrane contact sites. We propose that LysoKVCa serves as the perilysosomal Ca2+ effector to prime lysosomes for the refilling process. Consistently, genetic ablation or pharmacological inhibition of LysoKVCa, or abolition of its Ca2+ sensitivity, blocks refilling and maintenance of lysosomal Ca2+ stores, resulting in lysosomal cholesterol accumulation and a lysosome storage phenotype.
© 2017 Wang et al.
Figures





References
-
- Braun M., Ramracheya R., Bengtsson M., Zhang Q., Karanauskaite J., Partridge C., Johnson P.R., and Rorsman P.. 2008. Voltage-gated ion channels in human pancreatic beta-cells: Electrophysiological characterization and role in insulin secretion. Diabetes. 57:1618–1628. 10.2337/db07-0991 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

