GSK-3β Homolog Rim11 and the Histone Deacetylase Complex Ume6-Sin3-Rpd3 Are Involved in Replication Stress Response Caused by Defects in Dna2
- PMID: 28468907
- PMCID: PMC5499189
- DOI: 10.1534/genetics.116.198671
GSK-3β Homolog Rim11 and the Histone Deacetylase Complex Ume6-Sin3-Rpd3 Are Involved in Replication Stress Response Caused by Defects in Dna2
Abstract
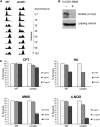
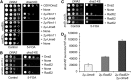
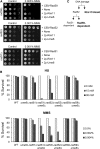
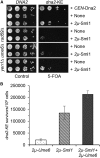
Lagging strand synthesis is mechanistically far more complicated than leading strand synthesis because it involves multistep processes and requires considerably more enzymes and protein factors. Due to this complexity, multiple fail-safe factors are required to ensure successful replication of the lagging strand DNA. We attempted to identify novel factors that are required in the absence of the helicase activity of Dna2, an essential enzyme in Okazaki-fragment maturation. In this article, we identified Rim11, a GSK-3β-kinase homolog, as a multicopy suppressor of dna2 helicase-dead mutant (dna2-K1080E). Subsequent epistasis analysis revealed that Ume6 (a DNA binding protein, a downstream substrate of Rim11) also acted as a multicopy suppressor of the dna2 allele. We found that the interaction of Ume6 with the conserved histone deacetylase complex Sin3-Rpd3 and the catalytic activity of Rpd3 were indispensable for the observed suppression of the dna2 mutant. Moreover, multicopy suppression by Rim11/Ume6 requires the presence of sister-chromatid recombination mediated by Rad52/Rad59 proteins, but not vice versa. Interestingly, the overexpression of Rim11 or Ume6 also suppressed the MMS sensitivity of rad59Δ. We also showed that the lethality of dna2 helicase-dead mutant was attributed to checkpoint activation and that decreased levels of deoxynucleotide triphosphates (dNTPs) by overexpressing Sml1 (an inhibitor of ribonucleotide reductase) rescued the dna2 mutant. We also present evidence that indicates Rim11/Ume6 works independently but in parallel with that of checkpoint inhibition, dNTP regulation, and sister-chromatid recombination. In conclusion, our results establish Rim11, Ume6, the histone deacetylase complex Sin3-Rpd3 and Sml1 as new factors important in the events of faulty lagging strand synthesis.
Keywords: Dna2; GSK-3b; HDAC; checkpoint; genome instability.
Copyright © 2017 by the Genetics Society of America.
Figures




Similar articles
-
Rad52/Rad59-dependent recombination as a means to rectify faulty Okazaki fragment processing.J Biol Chem. 2014 May 23;289(21):15064-79. doi: 10.1074/jbc.M114.548388. Epub 2014 Apr 7. J Biol Chem. 2014. PMID: 24711454 Free PMC article.
-
The Rpd3-Sin3 histone deacetylase regulates replication timing and enables intra-S origin control in Saccharomyces cerevisiae.Mol Cell Biol. 2004 Jun;24(11):4769-80. doi: 10.1128/MCB.24.11.4769-4780.2004. Mol Cell Biol. 2004. PMID: 15143171 Free PMC article.
-
Loss of Sin3/Rpd3 histone deacetylase restores the DNA damage response in checkpoint-deficient strains of Saccharomyces cerevisiae.Mol Cell Biol. 2003 Jul;23(13):4522-31. doi: 10.1128/MCB.23.13.4522-4531.2003. Mol Cell Biol. 2003. PMID: 12808094 Free PMC article.
-
Yet another job for Dna2: Checkpoint activation.DNA Repair (Amst). 2015 Aug;32:17-23. doi: 10.1016/j.dnarep.2015.04.009. Epub 2015 May 1. DNA Repair (Amst). 2015. PMID: 25956863 Free PMC article. Review.
-
Limiting homologous recombination at stalled replication forks is essential for cell viability: DNA2 to the rescue.Curr Genet. 2020 Dec;66(6):1085-1092. doi: 10.1007/s00294-020-01106-7. Epub 2020 Sep 9. Curr Genet. 2020. PMID: 32909097 Free PMC article. Review.
Cited by
-
The secondary-structured DNA-binding activity of Dna2 endonuclease/helicase is critical to cell growth under replication stress.FEBS J. 2021 Feb;288(4):1224-1242. doi: 10.1111/febs.15475. Epub 2020 Jul 19. FEBS J. 2021. PMID: 32638513 Free PMC article.
-
Yeast Rim11 kinase responds to glutathione-induced stress by regulating the transcription of phospholipid biosynthetic genes.Mol Biol Cell. 2024 Jan 1;35(1):ar8. doi: 10.1091/mbc.E23-03-0116. Epub 2023 Nov 8. Mol Biol Cell. 2024. PMID: 37938929 Free PMC article.
-
A Putative Zn(II)2Cys6-Type Transcription Factor FpUme18 Is Required for Development, Conidiation, Cell Wall Integrity, Endocytosis and Full Virulence in Fusarium pseudograminearum.Int J Mol Sci. 2023 Jul 1;24(13):10987. doi: 10.3390/ijms241310987. Int J Mol Sci. 2023. PMID: 37446163 Free PMC article.
References
-
- Bae S. H., Seo Y. S., 2000. Characterization of the enzymatic properties of the yeast dna2 Helicase/endonuclease suggests a new model for Okazaki fragment processing. J. Biol. Chem. 275: 38022–38031. - PubMed
-
- Bae S. H., Bae K. H., Kim J. A., Seo Y. S., 2001a RPA governs endonuclease switching during processing of Okazaki fragments in eukaryotes. Nature 412: 456–461. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

