Form and function of the teleost lateral line revealed using three-dimensional imaging and computational fluid dynamics
- PMID: 28468922
- PMCID: PMC5454279
- DOI: 10.1098/rsif.2016.0898
Form and function of the teleost lateral line revealed using three-dimensional imaging and computational fluid dynamics
Abstract
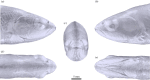
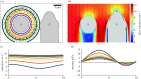
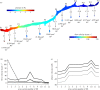
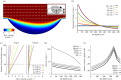
Fishes sense weak water motion using the lateral line. Among the thousands of described fish species, this organ may differ in size, shape and distribution of individual mechanoreceptors or lateral line canals. The reasons for this diversity remain unclear, but are very likely related to habitat preferences. To better understand the performance of the organ in natural hydrodynamic surroundings, various three-dimensional imaging datasets of the cephalic lateral line were gathered using Leuciscus idus as representative freshwater teleost. These data are employed to simulate hydrodynamic phenomena around the head and within lateral line canals. The results show that changes in canal dimensions alter the absolute stimulation amplitudes, but have little effect on the relation between bulk water flow and higher frequency signals. By contrast, depressions in the skin known as epidermal pits reduce bulk flow stimulation and increase the ratio between higher-frequency signals and the background flow stimulus.
Keywords: Leuciscus idus; finite-element modelling; laser scanning; micro-computed tomography; morphometry; photogrammetry.
© 2017 The Author(s).
Conflict of interest statement
We declare we have no competing interests.
Figures






Similar articles
-
Function of lateral line canal morphology.Integr Zool. 2015 Jan;10(1):111-21. doi: 10.1111/1749-4877.12101. Integr Zool. 2015. PMID: 24920149
-
The lateral line receptor array of cyprinids from different habitats.J Morphol. 2014 Apr;275(4):357-70. doi: 10.1002/jmor.20219. Epub 2013 Oct 21. J Morphol. 2014. PMID: 24142903
-
The flow fields involved in hydrodynamic imaging by blind Mexican cave fish (Astyanax fasciatus). Part I: open water and heading towards a wall.J Exp Biol. 2010 Nov 15;213(Pt 22):3819-31. doi: 10.1242/jeb.040741. J Exp Biol. 2010. PMID: 21037061
-
Sensory ecology of the fish lateral-line system: Morphological and physiological adaptations for the perception of hydrodynamic stimuli.J Fish Biol. 2019 Jul;95(1):53-72. doi: 10.1111/jfb.13966. Epub 2019 May 7. J Fish Biol. 2019. PMID: 30873616 Review.
-
Lateral line system of fish.Integr Zool. 2009 Mar;4(1):13-25. doi: 10.1111/j.1749-4877.2008.00131.x. Integr Zool. 2009. PMID: 21392273 Review.
Cited by
-
Structural and functional evolution of the mechanosensory lateral line system of fishesa).J Acoust Soc Am. 2023 Dec 1;154(6):3526-3542. doi: 10.1121/10.0022565. J Acoust Soc Am. 2023. PMID: 38171014 Free PMC article.
-
Canal neuromast position prefigures developmental patterning of the suborbital bone series in Astyanax cave- and surface-dwelling fish.Dev Biol. 2018 Sep 15;441(2):252-261. doi: 10.1016/j.ydbio.2018.04.001. Epub 2018 Apr 6. Dev Biol. 2018. PMID: 29630866 Free PMC article.
-
Head width influences flow sensing by the lateral line canal system in fishes.J Exp Biol. 2018 Oct 29;221(Pt 21):jeb180877. doi: 10.1242/jeb.180877. J Exp Biol. 2018. PMID: 30194249 Free PMC article.
-
Behavior, Electrophysiology, and Robotics Experiments to Study Lateral Line Sensing in Fishes.Integr Comp Biol. 2018 Nov 1;58(5):874-883. doi: 10.1093/icb/icy066. Integr Comp Biol. 2018. PMID: 29982706 Free PMC article. Review.
-
Synchrotron microtomography applied to the volumetric analysis of internal structures of Thoropa miliaris tadpoles.Sci Rep. 2020 Nov 3;10(1):18934. doi: 10.1038/s41598-020-75993-8. Sci Rep. 2020. PMID: 33144603 Free PMC article.
References
-
- Dijkgraaf S. 1933. Untersuchungen über die Funktion der Seitenorgane an Fischen. Z. Physiol. 20, 162–214. (10.1007/bf00340757) - DOI
-
- Beckmann M, Erös T, Schmitz A, Bleckmann H. 2010. Number and distribution of superficial neuromasts in twelve common European cypriniform fishes and their relationship to habitat occurrence. Int. Rev. Hydrobiol. 95, 273–284. (10.1002/iroh.200911185) - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

