ENaC activity is regulated by calpain-2 proteolysis of MARCKS proteins
- PMID: 28468944
- PMCID: PMC5538800
- DOI: 10.1152/ajpcell.00244.2016
ENaC activity is regulated by calpain-2 proteolysis of MARCKS proteins
Abstract
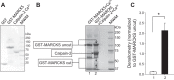
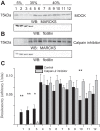
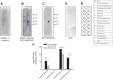
We previously demonstrated a role for the myristoylated alanine-rich C kinase substrate (MARCKS) to serve as an adaptor protein in the anionic phospholipid phosphate-dependent regulation of the epithelial sodium channel (ENaC). Both MARCKS and ENaC are regulated by proteolysis. Calpains are a family of ubiquitously expressed intracellular Ca2+-dependent cysteine proteases involved in signal transduction. Here we examine the role of calpain-2 in regulating MARCKS and ENaC in cultured renal epithelial cells and in the mouse kidney. Using recombinant fusion proteins, we show that MARCKS, but not the ENaC subunits, are a substrate of calpain-2 in the presence of Ca2+ Pharmacological inhibition of calpain-2 alters MARCKS protein expression in light-density sucrose gradient fractions from cell lysates of mouse cortical collecting duct cells. Calpain-dependent cleaved products of MARCKS are detectable in cultured renal cells. Ca2+ mobilization and calpain-2 inhibition decrease the association between ENaC and MARCKS. The inhibition of calpain-2 reduces ENaC activity as demonstrated by single-channel patch-clamp recordings and transepithelial current measurements. These results suggest that calpain-2 proteolysis of MARCKS promotes its interaction with lipids and ENaC at the plasma membrane to allow for the phosphatidylinositol 4,5-bisphosphate (PIP2)-dependent regulation of ENaC activity in the kidney.
Keywords: calcium; calpain; epithelial sodium channel; myristoylated alanine-rich C kinase substrate; protein kinase C; proteolysis.
Copyright © 2017 the American Physiological Society.
Figures







Similar articles
-
Calmodulin and CaMKII modulate ENaC activity by regulating the association of MARCKS and the cytoskeleton with the apical membrane.Am J Physiol Renal Physiol. 2015 Sep 1;309(5):F456-63. doi: 10.1152/ajprenal.00631.2014. Epub 2015 Jul 1. Am J Physiol Renal Physiol. 2015. PMID: 26136560 Free PMC article.
-
Phosphatidylinositol phosphate-dependent regulation of Xenopus ENaC by MARCKS protein.Am J Physiol Renal Physiol. 2012 Sep 15;303(6):F800-11. doi: 10.1152/ajprenal.00703.2011. Epub 2012 Jul 11. Am J Physiol Renal Physiol. 2012. PMID: 22791334 Free PMC article.
-
Phosphatidylinositol 4-phosphate 5-kinase reduces cell surface expression of the epithelial sodium channel (ENaC) in cultured collecting duct cells.J Biol Chem. 2007 Dec 14;282(50):36534-42. doi: 10.1074/jbc.M703970200. Epub 2007 Oct 16. J Biol Chem. 2007. PMID: 17940289
-
Regulation of the epithelial sodium channel (ENaC) by membrane trafficking.Biochim Biophys Acta. 2010 Dec;1802(12):1166-77. doi: 10.1016/j.bbadis.2010.03.010. Epub 2010 Mar 27. Biochim Biophys Acta. 2010. PMID: 20347969 Free PMC article. Review.
-
Membrane Surface Charge, Phospholipids, and Protein Localization.Rev Physiol Biochem Pharmacol. 2025;187:89-101. doi: 10.1007/978-3-031-68827-0_9. Rev Physiol Biochem Pharmacol. 2025. PMID: 39838010 Review.
Cited by
-
Direct and indirect inhibition of the circadian clock protein Per1: effects on ENaC and blood pressure.Am J Physiol Renal Physiol. 2019 May 1;316(5):F807-F813. doi: 10.1152/ajprenal.00408.2018. Epub 2019 Feb 13. Am J Physiol Renal Physiol. 2019. PMID: 30759025 Free PMC article.
-
Alpha 1-antitrypsin mitigates salt-sensitive hypertension in juvenile mice by reducing diacylglycerol concentrations and protein kinase C activity in kidney membranes.Front Mol Biosci. 2025 Jan 20;11:1485506. doi: 10.3389/fmolb.2024.1485506. eCollection 2024. Front Mol Biosci. 2025. PMID: 39902374 Free PMC article.
-
Metformin Alleviates Diabetes-Associated Hypertension by Attenuating the Renal Epithelial Sodium Channel.Biomedicines. 2023 Jan 21;11(2):305. doi: 10.3390/biomedicines11020305. Biomedicines. 2023. PMID: 36830842 Free PMC article.
-
Mycobacterium avium inhibits protein kinase C and MARCKS phosphorylation in human cystic fibrosis and non-cystic fibrosis cells.PLoS One. 2024 Oct 16;19(10):e0308299. doi: 10.1371/journal.pone.0308299. eCollection 2024. PLoS One. 2024. PMID: 39413095 Free PMC article.
-
PIEZO1 is a distal nephron mechanosensor and is required for flow-induced K+ secretion.J Clin Invest. 2024 Mar 1;134(5):e174806. doi: 10.1172/JCI174806. J Clin Invest. 2024. PMID: 38426496 Free PMC article.
References
-
- Alli AA, Bao HF, Liu BC, Yu L, Aldrugh S, Montgomery DS, Ma HP, Eaton DC. Calmodulin and CaMKII modulate ENaC activity by regulating the association of MARCKS and the cytoskeleton with the apical membrane. Am J Physiol Renal Physiol 309: F456–F463, 2015. doi:10.1152/ajprenal.00631.2014. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

