Luminal ANG II is internalized as a complex with AT1R/AT2R heterodimers to target endoplasmic reticulum in LLC-PK1 cells
- PMID: 28468964
- PMCID: PMC5582892
- DOI: 10.1152/ajprenal.00261.2016
Luminal ANG II is internalized as a complex with AT1R/AT2R heterodimers to target endoplasmic reticulum in LLC-PK1 cells
Abstract
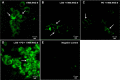
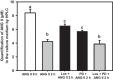
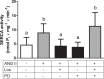
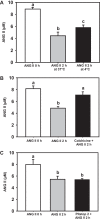
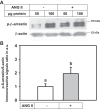
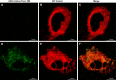
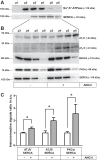
ANG II has many biological effects in renal physiology, particularly in Ca2+ handling in the regulation of fluid and solute reabsorption. It involves the systemic endocrine renin-angiotensin system (RAS), but tissue and intracrine ANG II are also known. We have shown that ANG II induces heterodimerization of its AT1 and AT2 receptors (AT1R and AT2R) to stimulate sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) activity. Thus, we investigated whether ANG II-AT1R/AT2R complex is formed and internalized, and also examined the intracellular localization of this complex to determine how its effect might be exerted on renal intracrine RAS. Living cell imaging of LLC-PK1 cells, quantification of extracellular ANG II, and use of the receptor antagonists, losartan and PD123319, showed that ANG II is internalized with AT1R/AT2R heterodimers as a complex in a microtubule-dependent and clathrin-independent manner, since colchicine-but not Pitstop2-blocked this process. This result was confirmed by an increase of β-arrestin phosphorylation after ANG II treatment, clathrin-mediated endocytosis being dependent on dephosphorylation of β-arrestin. Internalized ANG II colocalized with an endoplasmic reticulum (ER) marker and increased levels of AT1R, AT2R, and PKCα in ER-enriched membrane fractions. This novel evidence suggests the internalization of an ANG II-AT1/AT2 complex to target ER, where it might trigger intracellular Ca2+ responses.
Keywords: ANG II-AT1R/AT2R complex; ANG II-AT1R/AT2R internalization; LLC-PK1 luminal membranes; endoplasmic reticulum; microtubule-dependent clathrin-independent endocytosis.
Copyright © 2017 the American Physiological Society.
Figures

Similar articles
-
Exposure of luminal membranes of LLC-PK1 cells to ANG II induces dimerization of AT1/AT2 receptors to activate SERCA and to promote Ca2+ mobilization.Am J Physiol Renal Physiol. 2012 Apr 1;302(7):F875-83. doi: 10.1152/ajprenal.00381.2011. Epub 2012 Jan 4. Am J Physiol Renal Physiol. 2012. PMID: 22218590
-
Angiotensin II signaling via type 2 receptors in a human model of vascular hyporeactivity: implications for hypertension.J Hypertens. 2010 Jan;28(1):111-8. doi: 10.1097/HJH.0b013e328332b738. J Hypertens. 2010. PMID: 19797979
-
Angiotensin II type 2 receptors contribute to vascular responses in spontaneously hypertensive rats treated with angiotensin II type 1 receptor antagonists.Am J Hypertens. 2005 Apr;18(4 Pt 1):493-9. doi: 10.1016/j.amjhyper.2004.11.007. Am J Hypertens. 2005. PMID: 15831358
-
Angiotensin II type 2 receptor (AT2R) in renal and cardiovascular disease.Clin Sci (Lond). 2016 Aug 1;130(15):1307-26. doi: 10.1042/CS20160243. Clin Sci (Lond). 2016. PMID: 27358027 Review.
-
Is angiotensin-(3-4) (Val-Tyr), the shortest angiotensin II-derived peptide, opening new vistas on the renin-angiotensin system?J Renin Angiotensin Aldosterone Syst. 2017 Jan;18(1):1470320316689338. doi: 10.1177/1470320316689338. J Renin Angiotensin Aldosterone Syst. 2017. PMID: 28097883 Free PMC article. Review.
Cited by
-
Evidence for a Physiological Mitochondrial Angiotensin II System in the Kidney Proximal Tubules: Novel Roles of Mitochondrial Ang II/AT1a/O2- and Ang II/AT2/NO Signaling.Hypertension. 2020 Jul;76(1):121-132. doi: 10.1161/HYPERTENSIONAHA.119.13942. Epub 2020 Jun 1. Hypertension. 2020. PMID: 32475319 Free PMC article.
-
Angiotensin Receptors Heterodimerization and Trafficking: How Much Do They Influence Their Biological Function?Front Pharmacol. 2020 Aug 3;11:1179. doi: 10.3389/fphar.2020.01179. eCollection 2020. Front Pharmacol. 2020. PMID: 32848782 Free PMC article. Review.
-
Critical role of angiotensin II type 2 receptors in the control of mitochondrial and cardiac function in angiotensin II-preconditioned rat hearts.Pflugers Arch. 2018 Sep;470(9):1391-1403. doi: 10.1007/s00424-018-2153-9. Epub 2018 May 10. Pflugers Arch. 2018. PMID: 29748710 Free PMC article.
-
Dimerization of AT2 and Mas Receptors in Control of Blood Pressure.Curr Hypertens Rep. 2018 May 1;20(5):41. doi: 10.1007/s11906-018-0845-3. Curr Hypertens Rep. 2018. PMID: 29717388 Free PMC article. Review.
-
Mitochondrial angiotensin receptors and cardioprotective pathways.Am J Physiol Heart Circ Physiol. 2019 Jun 1;316(6):H1426-H1438. doi: 10.1152/ajpheart.00772.2018. Epub 2019 Apr 12. Am J Physiol Heart Circ Physiol. 2019. PMID: 30978131 Free PMC article. Review.
References
-
- Abadir PM, Foster DB, Crow M, Cooke CA, Rucker JJ, Jain A, Smith BJ, Burks TN, Cohn RD, Fedarko NS, Carey RM, O’Rourke B, Walston JD. Identification and characterization of a functional mitochondrial angiotensin system. Proc Natl Acad Sci USA 108: 14849–14854, 2011. doi: 10.1073/pnas.1101507108. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

