Mechanical regulation of cardiac fibroblast profibrotic phenotypes
- PMID: 28468977
- PMCID: PMC5541838
- DOI: 10.1091/mbc.E17-01-0014
Mechanical regulation of cardiac fibroblast profibrotic phenotypes
Abstract
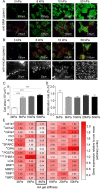
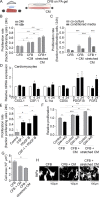
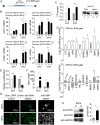
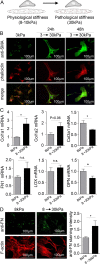
Cardiac fibrosis is a serious condition currently lacking effective treatments. It occurs as a result of cardiac fibroblast (CFB) activation and differentiation into myofibroblasts, characterized by proliferation, extracellular matrix (ECM) production and stiffening, and contraction due to the expression of smooth muscle α-actin. The mechanical properties of myocardium change regionally and over time after myocardial infarction (MI). Although mechanical cues are known to activate CFBs, it is unclear which specific mechanical stimuli regulate which specific phenotypic trait; thus we investigated these relationships using three in vitro models of CFB mechanical activation and found that 1) paracrine signaling from stretched cardiomyocytes induces CFB proliferation under mechanical conditions similar to those of the infarct border region; 2) direct stretch of CFBs mimicking the mechanical environment of the infarct region induces a synthetic phenotype with elevated ECM production; and 3) progressive matrix stiffening, modeling the mechanical effects of infarct scar maturation, causes smooth muscle α-actin fiber formation, up-regulation of collagen I, and down-regulation of collagen III. These findings suggest that myocyte stretch, fibroblast stretch, and matrix stiffening following MI may separately regulate different profibrotic traits of activated CFBs.
© 2017 Herum et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures

References
-
- Berry MF, Engler AJ, Woo YJ, Pirolli TJ, Bish LT, Jayasankar V, Morine KJ, Gardner TJ, Discher DE, Sweeney HL. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am J Physiol Heart Circ Physiol. 2006;290:H2196–H2203. - PubMed
-
- Camelliti P, Gallagher JO, Kohl P, McCulloch AD. Micropatterned cell cultures on elastic membranes as an in vitro model of myocardium. Nat Protoc. 2006;1:1379–1391. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

